Food Safety
Comments: No Comments
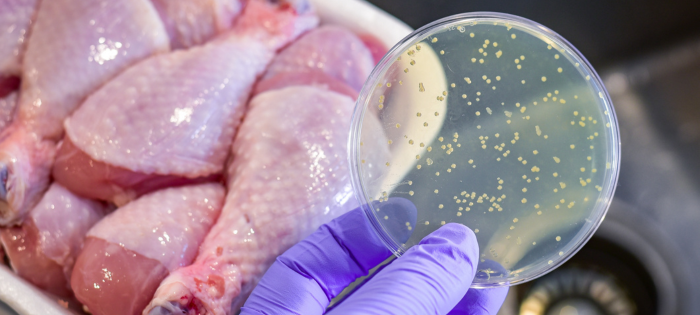
Salmonellosis (i.e., an infection with the bacteria Salmonella) is the second leading cause of foodborne illness in the U.S. The (CDC) Center for Disease Control estimates over 1.35 million illnesses, 26,500 hospitalizations, and nearly 420 deaths annually are attributed to Salmonella. It is estimated that over 23% of those illnesses are from eating chicken or turkey.
On October 19, 2021, the U.S. Department of Agriculture (USDA) launched a comprehensive effort to reduce Salmonella illnesses associated with poultry products. Since then, the Department has completed several activities to move closer to the national target of a 25% reduction in Salmonella illnesses (see below). One of the most significant actions came on July 29, 2024, when the USDA issued its proposed rule and determination to more effectively reduce Salmonella contamination and illnesses associated with raw poultry products.
Salmonella 101
Salmonella are bacteria that live in the intestinal tract of humans and animals and can contaminate some types of food, including raw fruits and vegetables and raw or undercooked chicken, turkey, beef, and eggs. Salmonella is transmitted by consuming contaminated food products. It can also be transferred when handling pets, particularly birds and reptiles.
Controlling Salmonella contamination is tricky for several reasons. There are approximately 2,500 serotypes (i.e., distinct strains) of Salmonella bacteria, and their risks differ across all production systems. Salmonella can survive under extreme conditions and adapt to its environment. It can also survive in most animals without causing disease in the animal. In fact, Salmonella can be found just about anywhere, including in food, water, soil, and the air we breathe.
Regulatory History
USDA’s most recent proposed rule and determination represents the culmination of the Food Safety and Inspection Service’s (FSIS) three-year effort to better control Salmonella rates in poultry and protect American consumers. The following significant events have led to this latest action:
- October 2022: FSIS announces a draft regulatory framework for a new strategy to control Salmonella in poultry products.
- June 2023: The USDA Agricultural Research Service (ARS) launches the Salmonella Grand Challenge, bringing together a group of scientists from different specialties to learn more about how and where Salmonella causes the highest risk to meat and poultry products.
- April 2024: FSIS publishes a final policy to declare Salmonella an adulterant in raw breaded chicken products when they exceed a threshold of 1 colony forming unit (CFU) per gram of Salmonella contamination. This represents the first time the USDA has labeled Salmonella as a contaminating adulterant in food, alongside certain types of E.coli.
Requirements of the Proposal Rule
The July 2024 proposed framework presents a systematic approach to addressing Salmonella contamination at the poultry slaughter and processing stages. It includes the first enforceable standards for Salmonella, something that has not been established until now because previously available tools and technology were not sufficient to track the bacteria.
The rule can be broken into the following two parts:
Enforceable Limits
The proposed rule establishes final standards to prevent raw chicken carcasses, chicken parts, ground chicken, and ground turkey products that contain any type of Salmonella at or above 10 CFU per gram/mL from entering the market. It also requires facilities to test for the presence of the following, which have been deemed Salmonella serotypes of public health significance:
| Product | Serotypes* |
| Chicken carcasses, chicken parts, comminuted (ground) chicken | – Enteritidis – Typhimurium – I4,[5],12:i |
| Comminuted (ground) turkey | – Hadar – Typhimurium – Muenchen |
If the bacteria exceed the established threshold of 10 CFU per gram/mL and contain any detectable level of at least one of the identified serotypes, the poultry cannot be sold, and the product lot would be subject to recall.
Monitoring, Sampling, and Testing
The final rule also requires poultry companies to establish microbial monitoring programs (MMPs) to identify and prevent pathogen contamination throughout the slaughter system. All poultry slaughter establishments would be required to develop, implement, and maintain written procedures to prevent contamination and maintain records documenting those procedures. More specifically, they must incorporate statistical process control (SPC) monitoring principles into their MMPs to monitor the quality of the manufacturing process. Facilities must also implement written corrective actions, including root cause analysis, when MMP results differ from the predefined criteria.
Finally, the proposed standard provides for a routine sampling and verification testing program for Salmonella in chicken parts, comminuted chicken, and comminuted turkey. FSIS would sample raw final products and analyze them for Salmonella levels and serotypes to determine whether the final product is adulterated. If test results detect Salmonella at a level of 10 CFU/mL or higher and at least one Salmonella serotype of public health significance, FSIS would consider products to be adulterated and the lot would be prohibited from entering commerce or a recall would be initiated.
Moving Forward
Setting limits on Salmonella levels has been difficult until now, because existing tools and technology were not sophisticated enough to track the bacteria. USDA Undersecretary for Food Safety Dr. Emilio Esteban has stated of this proposed rule, “It’s time to change our approach…We have the tools. We have the technology. We have the knowledge.”
The USDA also has the track record. The Department took similar action with E.coli in 1994 after deadly outbreaks tied to ground beef. As a result, the number of foodborne illnesses related to E.coli has fallen by more than 50%. The ultimate goal of the framework for Salmonella is similar—to get the U.S. closer to the national Healthy People 2030 target of a 25% reduction in salmonellosis cases.
USDA is accepting comments on the proposed final regulatory framework through September 29, 2024.
