Management Systems – Back to Basics
A management system is the organizing framework that enables companies to achieve and sustain their operational and business objectives through a process of continuous improvement. A management system is designed to identify and manage risks—safety, environmental, quality, business continuity, food safety (and many others)—through an organized set of policies, procedures, practices, and resources that guide the enterprise and its activities to maximize business value.
The management system addresses:
- What is done and why
- How it is done and by whom
- How well it is being done
- How it is maintained and reviewed
- How it can be improved
Creating an Effective and Valuable Management System
Each company’s management system reflects its unique culture, vision, and values. To be effective and valuable, the management system must be tailored and focused on how it can enhance the business performance of the organization. It must also be:
- Useful to people in the operations
- Intuitive—organized the way operations people think
- Flexible—making use of methods and tools as they are developed and documented
- Valuable from the outset—addressing the most critical risks and processes
- Linked to the business of the business (not “pasted on”), with ownership at the operational level
- A means to better align operational quality, safety, and environment with the business
Attributes of an effective management system are senior management expectations and guidance coupled with employee engagement. Importantly, a management system involves a continual cycle of planning, implementing, reviewing, and improving the way in which safety, quality, and environmental obligations and objectives are met. In its simplest form, this involves implementing the Plan, Do, Check, Act/Adjust (P-D-C-A) cycle for continuous improvement.
Auditing for Ongoing Compliance
The connection between management systems and compliance is vital in avoiding recurring compliance issues and in reducing variation in compliance performance. In fact, reliable and effective regulatory compliance is commonly an outcome of consistent and reliable implementation of a management system.
Conducting periodic audits is a practical way to test a management system’s implementation maturity and effectiveness. One of the many advantages of audits is that they help identify gaps so that corrective/preventive actions can be put into place and then sustained and improved through the management system.
Audits also help companies with continuous improvement initiatives; properly developed audit programs help measure results over time. To achieve best value, audits should emphasize finding patterns that can yield opportunities for learning and continual improvement, rather than “gotchas” for exceptions that are discovered.
Management System Standards
Several options are available for structuring management systems, whether they are certified by third-party registrars and auditors, self-certified, or used as internal guidance and for potential certification readiness.
The International Organization for Standardization (ISO) standards are some of the most commonly applied. The ISO standards for quality (ISO 9001), environment (ISO 14001), health & safety (OHSAS 18001), business continuity (ISO 22301), and food safety (FSSC 22000) have consistent elements, allowing organizations to more easily align their various management systems. Aligned management systems help companies to achieve improved and more reliable quality, environmental, and health & safety performance, while adding measurable business value.
Certification
Companies can become certified to each of the standards discussed above. Certification has a number of benefits, including the following:
- Meet customer or supply chain requirements
- Use outside drivers to maintain management system process discipline (e.g., periodic risk assessment, document management, compliance evaluation, internal audits, management review)
- Take advantage of third-party assessment and recommendations
- Improve standing with regulatory agencies (e.g., USEPA, OSHA, FDA, and state programs)
- Demonstrate the application of industry best practice in the event of incidents/accidents requiring defense of practices
However, if there is no market or other business driver, certification can lead to unnecessary additional cost and effort regarding management system development. Certification in itself does not mean improved performance—management system structure, operation, and management commitment determine that.
Business Value
There are a number of reasons to implement a management system. A properly designed and implemented management system brings value to organizations in a number of ways:
- Risk management
- Identify risks
- Set priorities for improvement, measurement, and reporting
- Provide great opportunity to identify, share, and learn best practices, while recognizing operational differences
- Protection of people
- Send people home the way they arrived at work
- Protect the public and the environment
- Compliance assurance
- Improve and sustain regulatory compliance
- Business value
- Continually improve quality, environmental, and safety performance across the organization (employee, public, equipment, infrastructure)
- Reduce incident costs and accrued liabilities
- Protect assets
- Reliability
- Assure processes, methods, and practices are in place, documented, and consistently applied
- Reduce variability in processes and performance
- Employee engagement
- Help employees to find and use current versions of all procedures and documents
- Provide a ready reference for field management to structure location-specific procedures
- Enable the effective transfer of standards, methods, and know-how in employee training, new job assignments, and promotions

Overview of FSMA for Chemical Distributors
Join NACD and Kestrel on Thursday, April 4 at 12:00 p.m. (EDT) for a webinar on the U.S. Food and Drug Administration’s (FDA) Food Safety Modernization Act (FSMA). During this webinar, Kestrel’s Roberto Bellavia, Principal in Food Safety Compliance, will provide an overview of FSMA rules applicable to the chemical ingredients industry, focusing on the receiving, storage, production, blending, and transportation of these products. Register here.
This webinar is a precursor to an upcoming in-person NACD regulatory workshop during which Kestrel will cover the FSMA regulations in-depth. This workshop will take place on June 12-13 in Oak Brook, Illinois, immediately following the Central Region Meeting. Registration for the workshop is forthcoming.

Comments: No Comments
Top Reasons to Pursue a Food Safety Management System
Designing and implementing a compliant Food Safety Management System (FSMS) can help organizations improve in many areas beyond the system’s defined tasks. It is critical for management to align the food safety objectives with the business needs for a successful and meaningful program implementation. Here are some of the top reasons why companies that work in the food industry may want to pursue developing and implementing an FSMS:
10. Identify and categorize the organization’s food safety risks.
Once this information is known, management can prioritize and decide how to eliminate or reduce business risks and liabilities to acceptable levels. These risks are often better controlled through strict management accounting. As a bonus, employees will become more attuned to thinking about risks and helping management improve overall operations.
9. Develop work instructions and/or procedures to guide employees’ actions and to ensure that each food safety task is completed in a disciplined manner and approved by management.
This will reduce the risk to an organization of an employee accidentally making a food safety mistake that causes the employee or others to be harmed (or worse). It also reduces the company’s risk of government inspections, fines, poor public perception, and loss of business due to a possible recall.
8. Assure management that they, in fact, know and understand the regulatory food safety requirements that must be met daily.
These requirements can be a driver of continual improvement by ensuring that the company has up-to-date procedures and work instructions for employees to follow every day.
7. Develop meaningful goals and objectives that drive food safety performance improvements and possibly reduce additional costs.
Each business will have different goals and these goals will likely change each year. Goals assure continuous improvement in food safety performance for the business over time.
6. Create a strong training and educational program that stems from well-written procedures and work instructions and that clearly defines the company’s requirements.
A well-trained workforce is a motivated and happy workforce. Turnover is reduced, accidents and incidents decrease, and production efficiencies increase. Employees are very aware when an organization takes time to ensure that each job requested is completed in the safest manner possible.
5. Develop appropriate monitoring and measurement practices.
Once all food safety requirements (e.g., FSMA, USDA, GFSI) are known and understood, the organization will be able to gauge food safety performance based on scientific data and regulations, and then guide the organization’s actions in a direction of continuous improvement and compliance.
4. Verify the FSMS is functioning as designed and implemented.
By continuously auditing each food safety program and function, the organization will discover issues of concern and non-conformances prior to an incident or agency/certifying body finding. Routine, non-biased audits allow the company to choose a timeframe that will help improve the situation without undue influence by outsiders.
3. Monitor and trend issues of concern and/or non-conformance and the actions used to rectify them through a fully functioning corrective/preventive action program.
As employees watch management fix problems, they will learn that management is concerned about continuous improvement. This will prompt employees to start making their own improvement suggestions. These suggestions will further drive improvement in areas outside the original FSMS.
2. Evaluate the business model and the FSMS in a holistic fashion.
By using this self-reflection and identifying improvement opportunities, management can direct responsibilities for improvement actions across many departments of the company. Each of these improvement opportunities has the potential to help the bottom line and reduce the possibility of a food safety liability now or in the future.
1. Know that the company has done everything to maintain the business in a manner that meets all food safety rules and regulations.
The last and most important benefit for an organization that goes through the process of designing and implementing a compliant FSMS is knowing that the organization has done everything possible to maintain its business in a manner that meets all food safety laws, regulations, and statutes every day the doors are open for business. To a business owner, that knowledge is priceless. This is how brands are built and how they maintain the promise of food safety to consumers.

Comments: No Comments
Be Our Guest at the Food Safety Consortium
On behalf of our team, Kestrel Management would like to invite you to attend the 6th Annual Food Safety Consortium Conference & Expo on Nov. 13-15 in Schaumburg, IL.
The Consortium is a premiere event for food safety education and networking—and we want to offer you the chance to visit us at the event (booth #119) for a discounted rate (see offer below).
You can accomplish more in two or three days at the Food Safety Consortium than you might otherwise achieve in weeks! Here are five ways the Food Safety Consortium will allow you to enhance your business:
- Get expert advice on specific challenges faced by your business.
- Listen to insights from thought leaders & innovators.
- Stay up-to-date with emerging or changing trends.
- Upgrade your skills, knowledge and on-the-job effectiveness.
- Gain new ideas and insights to grow your business.
Come see Kestrel at booth #119. When you register, use our discount code Cubs and receive a 20% discount off registration.
Our team is proud to be part of the Food Safety Consortium and hope to see you there!

Comments: No Comments
The Four “A’s” of Food Defense
When looking at FSMA, it’s important to look at what we should be doing in industry under FSMA’s prevention scheme. FDA seeks for companies to assess risk and implement preventive controls on a broad basis. Thinking about risk-based strategies, whether in the supply chain, internal systems, or whether you are a grower or an importer, is key for any food company when planning for the future.
From Reactive to Proactive
With the FSMA rules, FDA has moved from reactive to proactive. Preventive strategies are the essence of FSMA. Proactively creating or updating a food defense and safety plan is the first step to ensure compliance.
The four “A’s” of food defense, as outlined below, provide a methodology for building a proactive and comprehensive food defense program.
Step 1: Assess
Assess the risks throughout the supply chain, including to the origin of raw materials. Conduct a vulnerability assessment of weaknesses and critical control points to identify where someone could attempt product adulteration. The focus must be both inside and outside of company walls and extend to the source of materials and services within the supply chain for producers and distributors of food to the public.
Step 2: Access
Who has access to critical control points and food material risk areas? Pay close attention to the four key activity types that FDA has identified as particularly vulnerable to adulteration:
- Mixing and grinding activities that involve a high volume of food with a high potential for uniform mixing of a contaminant
- Ingredient handling with open access to the product stream
- Bulk liquid receiving and loading
- Liquid storage and handling, which is typically located in remote, isolated areas
Restrict access to these areas from suppliers, contractors, visitors, and most employees—limiting access to critical employees only. This provides a higher level of protection, and supports video and/or physical monitoring.
Step 3: Alerts
Alerts of intentional and unintentional food adulteration must be sent to the appropriate individuals, according to the documented food safety and defense program. Response time is critical. Every passing minute is a minute when more health risks could develop, leading to a greater chance of negative impacts on public safety and the related businesses.
Step 4: Audit
Auditing operational and regulatory compliance helps to ensure and maintain best food defense practices and provide documentation of compliance to regulators. FSMA promotes the safety of the U.S. food supply by focusing on prevention, rather than reactive response. Prevention is only as effective as the actual compliance processes put in place. Regular and random auditing, including remote video monitoring, provides evidence confirming that the appropriate preventive measures are taken and effective.
Taking a proactive approach to food defense that follows these four “A’s” will help meet a key requirement by ensuring that the organization is working to avoid the risks associated with food adulteration and contamination.

USTR Finalizes China 301 List 3 Tariffs
On Monday, September 17, 2018, the Office of the United States Trade Representative (USTR) released a list of approximately $200 billion worth of Chinese imports, including hundreds of chemicals, that will be subject to additional tariffs. The additional tariffs will be effective starting September 24, 2018, and initially will be in the amount of 10 percent. Starting January 1, 2019, the level of the additional tariffs will increase to 25 percent.
In the final list, the administration also removed nearly 300 items, but the Administration did not provide a specific list of products excluded. Included among the products removed from the proposed list are certain consumer electronics products, such as smart watches and Bluetooth devices; certain chemical inputs for manufactured goods, textiles and agriculture; certain health and safety products such as bicycle helmets, and child safety furniture such as car seats and playpens.
Individual companies may want to review the list to determine the status of Harmonized Tariff Schedule (HTS) codes of interest.

Assessing Risk Management Program Maturity
Maturity assessments are designed to tell an organization where it stands in a defined area and, correspondingly, what it needs to do in the future to improve its systems and processes to meet the organization’s needs and expectations. Maturity assessments expose the strengths and weaknesses within an organization (or a program), and provide a roadmap for ongoing improvements.
Holistic Assessments
A thorough program maturity assessment involves building on a standard gap analysis to conduct a holistic evaluation of the existing program, including data review, interviews with key staff, and functional/field observations and validation.
Based on Kestrel’s experience, evaluating program maturity is best done by measuring the program’s structure and design, as well as the program’s implementation consistency across the organization. For the most part, a program’s design remains relatively unchanging, unless internal modifications are made to the system. Because of this static nature, a “snapshot” provides a reasonable assessment of the design maturity. While the design helps to inform operational effectiveness, the implementation/operational maturity model assesses how completely and consistently the program is functioning throughout the organization (i.e., how the program is designed to work vs. how it is working in practice).
Design Maturity
A design maturity model helps to evaluate strategies and policies, practices and procedures, organization and people, information for decision making, and systems and data according to the following levels of maturity:
- Level 1: Initial (crisis management) – Lack of alignment within the organization; undefined policies, goals, and objectives; poorly defined roles; lack of effective training; erratic program or project performance; lack of standardization in tools.
- Level 2: Repeatable (reactive management) – Limited alignment within the organization; lagging policies and plans; seldom known business impacts of actions; inconsistent company operations across functions; culture not focused on process; ineffective risk management; few useful program or project management and controls tools.
- Level 3: Defined (project management) – Moderate alignment across the organization; consistent plans and policies; formal change management system; somewhat defined and documented processes; moderate role clarity; proactive management for individual projects; standardized status reporting; data integrity may still be questionable.
- Level 4: Managed (program management) – Alignment across organization; consistent plans and policies; goals and objectives are known at all levels; process-oriented culture; formal processes with adequate documentation; strategies and forecasts inform processes; well-understood roles; metrics and controls applied to most processes; audits used for process improvements; good data integrity; programs, processes, and performance reviewed regularly.
- Level 5: Optimized (managing excellence) – Alignment from top to bottom of organization; business forecasts and plans guide activity; company culture is evident across the organization; risk management is structured and proactive; process-centered structure; focus on continuous improvement, training, coaching, mentoring; audits for continual improvement; emphasis on “best-in-class” methods.
A gap analysis can help compare the actual program components against best practice standards, as defined by the organization. At this point, assessment questions and criteria should be specifically tuned to assess the degree to which:
- Hazards and risks are identified, sized, and assessed
- Existing controls are adequate and effective
- Plans are in place to address risks not adequately covered by existing controls
- Plans and controls are resourced and implemented
- Controls are documented and operationalized across applicable functions and work units
- Personnel know and understand the controls and expectations and are engaged in their design and improvement
- Controls are being monitored with appropriate metrics and compliance assurance
- Deficiencies are being addressed by corrective/preventive action
- Processes, controls, and performance are being reviewed by management for continual improvement
- Changed conditions are continually recognized and new risks identified and addressed
Implementation/Operational Maturity
The logical next step in the maturity assessment involves shifting focus from the program’s design to a maturity model that measures how well the program is operationalized, as well as the consistency of implementation across the entire organization. This is a measurement of how effectively the design (program static component) has enabled the desired, consistent practice (program dynamic component) within and across the company.
Under this model, the stage of maturity (i.e., initial, implementation in process, fully functional) is assessed in the following areas:
- Adequacy and effectiveness: demonstration of established processes and procedures with clarity of roles and responsibilities for managing key functions, addressing significant risks, and achieving performance requirements across operations
- Consistency: demonstration that established processes and procedures are fully applied and used across all applicable parts of the organization to achieve performance requirements
- Sustainability: demonstration of an established and ongoing method of review of performance indicators, processes, procedures, and practices in-place for the purpose of identifying and implementing measures to achieve continuing improvement of performance
This approach relies heavily on operational validation and seeking objective evidence of implementation maturity by performing functional and field observations and interviews across a representative sample of operations, including contractors.
Cultural Component
Performance within an organization is the combined result of culture, operational systems/controls, and human performance. Culture involves leadership, shared beliefs, expectations, attitudes, and policy about the desired behavior within a specific company. To some degree, culture alone can drive performance. However, without operational systems and controls, the effects of culture are limited and ultimately will not be sustained. Similarly, operational systems/controls (e.g., management processes, systems, and procedures) can improve performance, but these effects also are limited without the reinforcement of a strong culture. A robust culture with employee engagement, an effective management system, and appropriate and consistent human performance are equally critical.
A culture assessment incorporates an assessment of culture and program implementation status by performing interviews and surveys up, down, and across a representative sample of the company’s operations. Observations of company operations (field/facility/functional) should be done to verify and validate.
A culture assessment should evaluate key attributes of successful programs, including:
- Leadership
- Vision & Values
- Goals, Policies & Initiatives
- Organization & Structure
- Employee Engagement, Behaviors & Communications
- Resource Allocation & Performance Management
- Systems, Standards & Processes
- Metrics & Reporting
- Continually Learning Organization
- Audits & Assurance
Assessment and Evaluation
Data from document review, interviews, surveys, and field observations are then aggregated, analyzed, and evaluated. Identifying program gaps and issues enables a comparison of what must be improved or developed/added to what already exists. This information is often organized into the following categories:
- Policy and strategy refinements
- Process and procedure improvements
- Organizational and resource requirements
- Information for decision making
- Systems and data requirements
- Culture enhancement and development
From this information, it becomes possible to identify recommendations for program improvements. These recommendations should be integrated into a strategic action plan that outlines the long-term program vision, proposed activities, project sequencing, and milestones. The highest priority actions should be identified and planned to establish a foundation for continual improvement, and allow for a more proactive means of managing risks and program performance.

Audit Program Best Practices: Part 2
Audits provide an essential tool for improving and verifying compliance performance. As discussed in Part 1, there are a number of audit program elements and best practices that can help ensure a comprehensive audit program. Here are 12 more tips to put to use:
- Action item closure. Address repeat findings. Identify patterns and seek root cause analysis and sustainable corrections.
- Training. Training should be done throughout the entire organization, across all levels:
- Auditors are trained on both technical matters and program procedures.
- Management is trained on the overall program design, purpose, business impacts of findings, responsibilities, corrections, and improvements.
- Line operations are trained on compliance procedures and company policy/systems.
- Communications. Communications with management should be done routinely to discuss status, needs, performance, program improvements, and business impacts. Communications should be done in business language—with business impacts defined in terms of risks, costs, savings, avoided costs/capital expenditures, benefits. Those accountable for performance need to be provided information as close to “real time” as possible, and the Board of Directors should be informed routinely.
- Leadership philosophy. Senior management should exhibit top-down expectations for program excellence. EHSMS quality excellence goes hand-in-hand with operational and service quality excellence. Learning and continual improvement should be emphasized.
- Roles & responsibilities. Clear roles, responsibilities, and accountabilities need to be established. This includes top management understanding and embracing their roles/responsibilities. Owners of findings/fixes also must be clearly identified.
- Funding for corrective actions. Funding should be allocated to projects based on significance of risk exposure (i.e., systemic/preventive actions receive high priority). The process should incentivize proactive planning and expeditious resolution of significant problem areas and penalize recurrence or back-sliding on performance and lack of timely fixes.
- Performance measurement system. Audit goals and objectives should be nested with the company business goals, key performance objectives, and values. A balanced scorecard can display leading and lagging indicators. Metrics should be quantitative, indicative (not all-inclusive), and tied to their ability to influence. Performance measurements should be communicated and widely understood. Information from auditing (e.g., findings, patterns, trends, comparisons) and the status of corrective actions often are reported on compliance dashboards for management review.
- Degree of business integration. There should be a strong link between programs, procedures, and methods used in a quality management program—EHS activities should operate in patterns similar to core operations rather than as ancillary add-on duties. In addition, EHS should be involved in business planning and MOC. An EHSMS should be well-developed and designed for full business integration, and the audit program should feed critical information into the EHSMS.
- Accountability. Accountability and compensation must be clearly linked at a meaningful level. Use various award/recognition programs to offer incentives to line operations personnel for excellent EHS performance. Make disincentives and disciplinary consequences clear to discourage non-compliant activities.
- Deployment plan & schedule. Best practice combines the use of pilot facility audits, baseline audits (to design programs), tiered audits, and a continuous improvement model. Facility profiles are developed for all top priority facilities, including operational and EHS characteristics and regulatory and other requirements.
- Relation of audit program to EHSMS design & improvement objectives. The audit program should be fully interrelated with the EHSMS and feed critical information on systemic needs into the EHSMS design and review process. It addresses the “Evaluation of Compliance” element under EHSMS international standards (e.g., ISO 14001 and OHSAS 18001). Audit baseline helps identify common causes, systemic issues, and needed programs. The EHSMS addresses root causes and defines/improves preventive systems and helps integrate EHS with core operations. Audits further evaluate and confirm performance of EHSMS and guide continuous improvement.
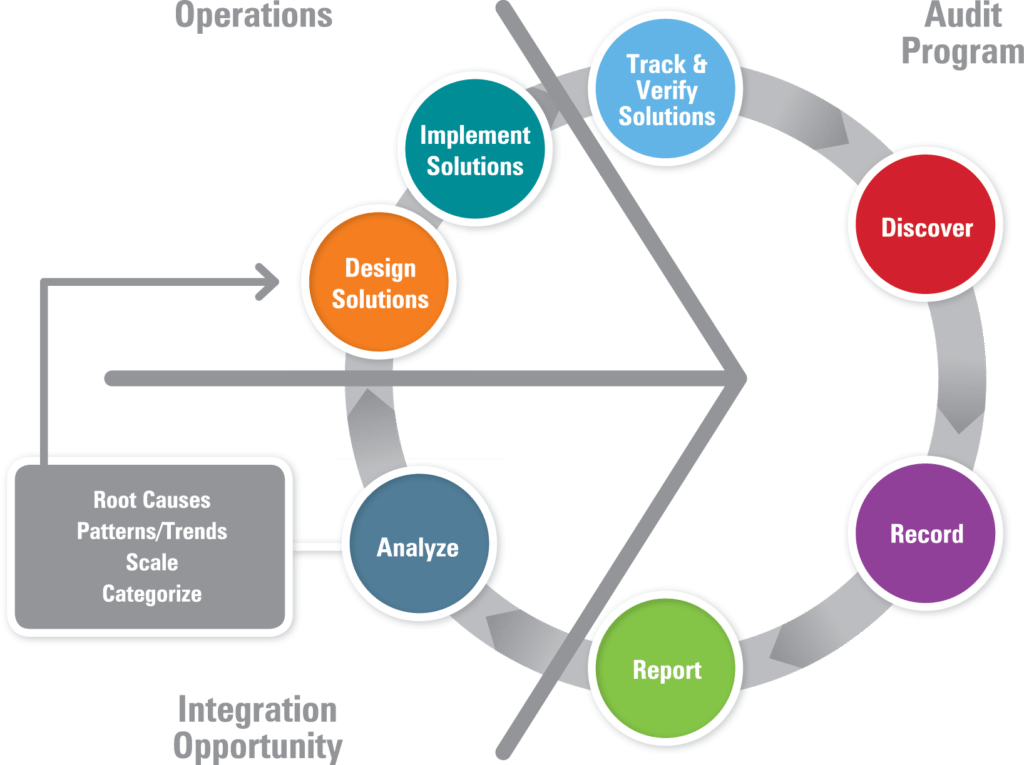
- Relation to best practices. Inventory best practices and share/transfer them as part of audit program results. Use best-in-class facilities as models and “problem sites” for improvement planning and training. The figure below illustrates an audit program that goes beyond the traditional “find it, fix it, find it, fix it” repetitive cycle to one that yields real understanding of root causes and patterns. In this model, if the issues can be categorized and are of wide scale, the design of solutions can lead to company-wide corrective and preventive measures. This same method can be used to capture and transfer best practices across the organization. They are sustained through the continual review and improvement cycle of an EHSMS and are verified by future audits.
Read the part 1 audit program best practices.

Audit Program Best Practices: Part 1
Audits provide an essential tool for improving and verifying compliance performance. Audits may be used to capture regulatory compliance status, management system conformance, adequacy of internal controls, potential risks, and best practices. An audit is typically part of a broader compliance assurance program and can cover some or all of the company’s legal obligations, policies, programs, and objectives.
Companies come in a variety of sizes with a range of different needs, so auditing standards remain fairly flexible. There are, however, a number of audit program elements and best practices that can help ensure a comprehensive audit program:
- Goals. Establishing goals enables recognition of broader issues and can lead to long-term preventive programs. This process allows the organization to get at the causes and focus on important systemic issues. It pushes and guides toward continuous improvement. Goal-setting further addresses the responsibilities and obligations of the Board of Directors for audit and oversight and elicits support from stakeholders.
- Scope. The scope of the audit should be limited initially (e.g., compliance and risk) to what is manageable and to what can be done very well, thereby producing performance improvement and a wider understanding and acceptance of objectives. As the program is developed and matures (e.g., Management Systems, company policy, operational integration), it can be expanded and, eventually, shift over time toward systems in place, prevention, efficiency, and best practices.
- Committed resources. Sufficient resources must be provided for staffing and training and then applied, as needed, to encourage a robust auditing program. Resources also should be applied to EHSMS design and continuous improvement. It is important to track the costs/benefits to compare the impacts and results of program improvements.
- Operational focus. All facilities need to be covered at the appropriate level, with emphasis based on potential EHS and business risks. The operational units/practices with the greatest risk should receive the greatest attention (e.g., the 80/20 Rule). Vendors/contractors and related operations that pose risks must be included as part of the program. For smaller, less complex and/or lower risk facilities, lower intensity focus can be justified. For example, relying more heavily on self-assessment and reporting of compliance and less on independent audits may provide better return on investment of assessment resources.
- Audit team. A significant portion of the audit program should be conducted by knowledgeable auditors (independent insiders, third parties, or a combination thereof) with clear independence from the operations being audited and from the direct chain of command. For organizational learning and to leverage compliance standards across facilities, it is good practice to vary at least one audit team member for each audit. Companies often enlist personnel from different facilities and with different expertise to audit other facilities. Periodic third-party audits further bring outside perspective and reduce tendencies toward “home-blindness”.
- Audit frequency. There are several levels of audit frequency, depending on the type of audit:
- Frequent: Operational (e.g., inspections, housekeeping, maintenance) – done as part of routine EHSMS day-to-day operational responsibilities
- Periodic: Compliance, systems, actions/projects – conducted annually/semi-annually
- As needed: For issue follow-up
- Infrequent: Comprehensive, independent – conducted every three to four years
- Differentiation methods. Differentiating identifies and distinguishes issues of greatest importance in terms of risk reduction and business performance improvement. The process for differentiating should be as clear and simple as possible; a system of priority rating and ranking is widely understood and agreed. The rating system can address severity levels, as well as probability levels, in addition to complexity/difficulty and length of time required for corrective actions.
- Legal protection. Attorney privilege for audit processes and reports is advisable where risk/liability are deemed significant, especially for third-party independent audits. To the extent possible, make the audit process and reports become management tools that guide continuous improvement. Organizations should follow due diligence elements of the USEPA audit policy.
- Procedures. Describe and document the audit process for consistent, efficient, effective, and reliable application. The best way to do this is to involve both auditors and those being audited in the procedure design. Audit procedures should be tailored to the specific facility/operation being audited. Documented procedures should be used to train both auditors and those accountable for operations being audited. Procedures can be launched using a pilot facility approach to allow for initial testing and fine-tuning. Keep procedures current and continually improve them based on practical application. Audits include document and record review (corporate and facility), interviews, and observations.
- Protocols & tools. Develop specific and targeted protocols that are tailored to operational characteristics and based on applicable regulations and requirements for the facility. Use “widely accepted or standard practice” as go-by tools to aid in developing protocols (e.g., ASTM site assessment standards; ISO 14010 audit guidance; audit protocols based on EPA, OSHA, MSHA, Canadian regulatory requirements; GEMI self-assessment tools; proprietary audit protocol/tools). As protocols are updated, the ability to evaluate continuous improvement trends must be maintained (i.e., trend analysis).
- Information management & analysis. Procedures should be well-defined, clear, and consistent to enable the organization to analyze trends, identify systemic causes, and pinpoint recurring problem areas. Analysis should prompt communication of issues and differentiation among findings based on significance. Audit reports should be issued in a predictable and timely manner. It is desirable to orient the audit program toward organizational learning and continual improvement, rather than a “gotcha” philosophy. “Open book” approaches help learning by letting facility managers know in advance what the audit protocols are and how the audits will be conducted.
- Verification & corrective action. Corrective actions require corporate review, top management-level attention and management accountability for timely completion. A robust root cause analysis helps to ensure not just correction/containment of the existing issue, but also preventive action to assure controls are in place to prevent the event from recurring. For example, if a drum is labeled incorrectly, the corrective action is to relabel that drum. A robust plan should also look for other drums than might be labeled incorrectly and to add and communicate an effective preventive action (e.g., training or posting signs showing a correctly labeled drum).
Read the part 2 audit program best practices.

10 Reasons to Implement a Management System
A management system is the framework that enables companies to achieve their operational and business objectives through a process of continuous improvement. In its simplest form, a management system implements the Plan, Do, Check, Act/Adjust cycle. Several choices are available for management systems (ISO is commonly applied), whether they are certified by third-party registrars and auditors, self-certified, or used as internal guidance and for potential certification readiness.
Business Benefits of a Well-Documented Management System
The connection between management systems and compliance is vital in avoiding recurring compliance issues and in reducing variation in compliance performance. In fact, reliable and effective regulatory compliance is commonly an outcome of consistent and reliable implementation of a management system.
Beyond that, there are a number of business reasons for implementing a well-documented management system (environmental, safety, quality, food safety, other) and associated support methods and tools:
- Establishes a common documented framework to achieve more consistent implementation of compliance policies and processes—addressing the eight core functions of compliance:
- Inventories
- Permits and authorizations
- Plans
- Training
- Practices in place
- Monitoring and inspection
- Records
- Reporting
- Provides clear methods and processes to identify and prioritize risks, set and monitor goals, communicate those risks to employees and management, and allocate the resources to mitigate them.
- Shifts from a command-and-control, centrally driven function to one that depends heavily on teamwork and implementation of a common system, taking into consideration the necessary local differences and building better know-how at the facility level.
- Establishes a common language for periodic calls and meetings among managers, facility managers, and executives, which yields better goal-setting, priority ranking, and allocation of resources to the areas with greatest risk or the greatest opportunity to add business value.
- Empowers facilities to take responsibility for processes and compliance performance without waiting to be told “what” and “how”.
- Enables better collaboration and communication across a distributed company with many locations.
- Enables the selection and implementation of a robust information system capable of tracking and reporting on common activities and performance metrics across the company.
- Employs a design and implementation process that builds company know-how, captures/retains institutional knowledge, and enables ongoing improvement without having to continually reinvent the wheel.
- Creates consistent processes and procedures that support personnel changes (e.g., transfers, promotions, retirements) and training of new personnel without causing disruption or gaps.
- Allows for more consistent oversight and governance, yielding higher predictability and reliability.