
Comments: No Comments
Food Safety Magazine Article: Food Remote Audits
Audits provide an essential tool for improving and verifying compliance performance. Audits may be used to capture regulatory compliance status (e.g., FDA, USDA); certification system conformance (e.g., FSSC 22000, SQF, IFS, BRC); and adequacy of internal controls, potential risks, and best practices.
Most regulations, standards, and certification programs require audits to be conducted with some established frequency. For many food companies, figuring out how to meet these audit requirements amongst travel restrictions, new company safety protocol, and government quarantines related to COVID-19 has presented a significant challenge.
The Online Alternative
Fortunately, the Global Food Safety Initiative (GFSI) and the benchmarked certification schemes have responded to this challenge, recognizing that online/remote/virtual audits can offer a viable alternative to onsite audits—even when companies are not operating in a pandemic.
Read KTL’s recent article in Food Safety Magazine about remote food safety auditing and best practices for doing it right.

Demonstrating Compliance in a Socially Distanced World
Don’t miss this free American Bar Association event on April 22, 2021 — Demonstrating Compliance in a Socially Distanced World: Virtual Auditing.
In the time of COVID-19, virtual auditing has become increasingly necessary and valuable to organizations as they seek to achieve environmental compliance while facing worldwide travel restrictions and remote work policies that have disrupted routine in-person audits. With this shift, comes the need for both regulated entities and regulators to develop new approaches and procedures to ensure the effectiveness of audits conducted remotely. Practitioners, including auditors and legal counsel, must consider new dynamics related to security, data protection, and audit integrity-on top of the usual audit considerations. This session will highlight some of these new challenges and provide real-world solutions to aid attendees form new practice skills to apply in the (virtual) field.
Panelists–including KTL’s Sarah Burton–will explore the new world of remote auditing, focusing on real-world solutions to the challenges that virtual auditing presents.

Comments: No Comments
Food Safety Tech Article: Integrated Food CMS
Managing the complexities of a management system is challenging for any food and beverage company, particularly for the team tasked with implementing the system throughout the organization. That is because every regulatory agency (e.g., FDA, USDA, OSHA, EPA) and voluntary certification (e.g., GFSI-benchmarked standards, gluten-free, organic, ISO) calls for companies to fulfill compliance requirements—many of which overlap. Supply chain and internal requirements can create further complications and confusion.
In today’s “New Era of Smarter Food Safety,” having a common system to organize, manage and track compliance offers an ideal solution. Dynamic tools are becoming available—systems that can manage employee training, pest control, laboratory testing, supply chain management tools, regulatory compliance and certification requirements, etc.
Unfortunately, these systems are often not set up to “talk” to each other, leaving company representatives to navigate many systems, databases, folders, and documents housed in many different locations.
The Solution: Compliance Management Systems
An integrated compliance management system (CMS) is intended to bring all these tools together to create one system that effectively manages compliance requirements, enables staff to carry out daily tasks and manage operations, and supports operational decision making by tracking and trending data that is collected daily by the team charged with implementation.
Read KTL’s recent article and case study in Food Safety Tech about how a CMS can be used to coordinate, organize, control, analyze, and visualize information to help organizations remain in compliance and operate efficiently.

Comments: 1 Comment
IFS Food V7: Creating a Food Safety Culture
The International Featured Standards (IFS) are “uniform food, product, and service standards that ensure certified companies produce a product or provide a service that complies with customer specifications, while continually working on process improvement.”
There are currently six standards under IFS, including the most recent IFS Food Standard Version 7 (IFS Food V7), which was issued in October 2020. Recognized by the Global Food Safety Initiative (GFSI) as a benchmarked standard for auditing food manufacturers, IFS Food focuses on ensuring food safety and quality.
V7 Changes
The new IFS Food V7 reduces the number of requirements by 15% and provides additional clarity for auditors when performing an IFS assessment through the following changes:
- Risk-based and less prescriptive approach that allows for customized solutions for company-specific risks and hazards
- New wording that changes “audit” to “assessment” to create consistency with ISO/IEC 17065
- New structure that emphasizes onsite evaluation over documentation
- Better defined scoring system and a more clearly structured assessment report
- Unannounced assessments (every third certification)
- Checklist that aligns with the GFSI Benchmarking Requirements Version 2020, Food Safety Modernization Act (FSMA), and European Union (EU) regulations
- Incorporation of food safety culture into assessments
IFS Food V7 is scheduled for implementation with audits beginning March 1, 2021 and will become mandatory for all certified companies on July 1, 2021.
Focus on Food Safety Culture
One notable change with IFS Food V7 is the incorporation of food safety culture into requirements. This is in line with the addition of food safety culture into GFSI Benchmarking Requirements Version 2020. It also relates directly to one of the core elements of the U.S. Food & Drug Administration’s (FDA) New Era of Smarter Food Safety, which promotes food safety culture throughout the food system.
Per IFS, “Food safety culture refers to shared values, beliefs, and norms that affect mindset and behaviour toward food safety in, across, and throughout an organization.”
Informally, food safety culture can be thought of as “the way we do things around here” when it comes to food safety practices. An organization’s food safety culture is ultimately reflected in the way food safety is managed in the workplace. A strong food safety culture creates an atmosphere where everyone in the organization is aware of and helps to prevent any process and/or operational issues and deviations that my impact the safety and/or quality of their products.
Characteristics of a Strong Food Safety Culture
Per IFS Food V7, food safety culture should be driven by four primary elements:
- Communication about food safety policies and responsibilities
- Training
- Employee feedback
- Performance measurement
Best-in-class food safety cultures have robust systems in place to ensure that each of these elements, among others, is well-functioning and fully ingrained into the standard practices of the organization. KTL has found that organizations with strong food safety cultures typically exhibit many of the following attributes:
- Communication. Communication is most effective when it comprises a combination of top-down and bottom-up interaction. All levels of management (senior, middle, supervisory) are responsible for clearly communicating to the workers who carry out the company’s mission. It is equally important that workers provide feedback on a practical level about what’s working and what’s not.
- Commitment. When it comes to food safety, actions truly speak louder than words. A lack of commitment, as demonstrated by action (or lack thereof), comes across loud and clear to staff.
- Caring. Caring involves showing concern for the personal safety of individuals (employees and consumers), not just making a commitment to the overall idea of food safety.
- Cooperation. Cooperation means working together to develop a strong food safety program. It means management seeks feedback from workers about food safety issues—and uses that feedback to make improvements.
- Coaching. Coaching each other—peer to peer, supervisor to employee, even employee to management—is an important way to keep everyone on track, generate constructive criticism, and foster a truly collaborative atmosphere regarding food safety.
- Procedures. There should be documented, clear procedures for every task. This not only prevents disagreement about what is required, it also shows commitment when things are put in writing.
- Training. Training is a more formal, documented process for ensuring that employees follow food safety processes and procedures and feel prepared to do their jobs.
- Tools. All equipment and tools should be in good repair and functioning as designed. Inadequate equipment/tools directly impact food safety/protection and indirectly impact perception of management commitment.
- Personnel. There must be enough qualified workers to perform each task. The company must not sacrifice food safety or quality because of being understaffed (i.e., requiring shortcuts/overtime to meet production goals).
- Trust. Trust in the food safety program, in senior management, and in each other is built when each of these characteristics is present and treated as a company-wide priority.
Planning for Change
For companies that are IFS-certified, now is the ideal time to assess current IFS Food program elements; identify improvements that are internally desirable and required by the new standard; and implement those updates that will create a strong food safety culture and make the IFS Food program more useful to the business. This can be done through a series of phases to ensure adoption throughout the organization.
- Phase 1: IFS Food Internal Assessment – Review existing IFS food programs, processes, and procedures; document management systems; and employee training tools and programs to identify those need areas in need of updates, development, and/or implementation to meet the requirements of IFS Food V7.
- Phase 2: IFS Food Program Updates – Based on the assessment, develop a plan for updating the IFS Food certification program, including major activities, key milestones, and expected outcomes. This may include updating/developing IFS Food programs, processes, procedures, and training with missing V7 requirements and incorporating new food safety culture requirements.
- Phase 3: Training – To ensure staff are prepared to implement and sustain the updated IFS Food V7 program, staff must be trained on applicable requirements; specific plans, procedures, and GMPs developed to achieve compliance; and the certification roadmap to prepare for future assessments.
Following this plan now will help companies ensure they maintain their IFS Food certification when assessments begin under IFS Food V7 in March 2021.
Resources
The IFS website has several comprehensive resources available to assist facilities and auditors in understanding the IFS Food V7 changes and requirements, including:

Environment / Food Safety / Quality / Safety / Technology Enabled Business Solutions
Comments: No Comments
From Paper Management to Digital Management
Virtually every regulatory agency (e.g., EPA, OSHA, FDA, USDA) and voluntary certification standard (e.g., ISO, GFSI, organic) has compliance requirements that call for companies to fulfill several common compliance activities. KTL has outlined eight compliance functions that can be instrumental in improving a company’s capability to comply. One very important compliance function involves records and document management.
Records provide documentation of what has been done related to compliance—current inventories, plans, management systems, training, inspections, and monitoring required for a given compliance or certification program. Each program typically has recordkeeping, records maintenance, and retention requirements specified by type. Having a good records management system is essential for maintaining the vast number of documents required by regulations and standards, particularly since some, like OSHA have retention cycles for as long as 30 years.
Moving Away from Paper Recordkeeping
Organizing and maintaining the records can create challenges—where to store them, security levels, remote and local accessibility, etc. Supply chain requirements can further add to the cumbersome workload of collecting, reviewing, and sharing documents and information.
Companies have been keeping records and documents in binders and file cabinets for years. And while that system can work, many dynamic tools are available to alleviate some of these challenges and support organizational decision-making. A document management system can help create:
- Process and document standardization
- Central and secure storage, organization, and access to documents and records locally or remotely
- Improved document searchability and accessibility
- Enhanced workflows for approving and completing tasks involving documents
- Easy access to documents for audits and clear audit trail, particularly for remote audits
- Version control and history
- Reduced paperwork
- Higher quality data due to reduced human error
- Improved collaboration
- Improved security of sensitive documents
All of which lead to consistent, efficient, and reliable compliance performance.
Transitioning Your Records
Transitioning from a paper-based recordkeeping system to an electronic document management system can seem overwhelming, particularly given the sheer volume of documents some organizations have. However, following a step-by-step approach—and considering the desired end product from the start—can help ensure that organizations end up with a system that will function well within the business context and provide ongoing compliance efficiency.
Step 1. Assess Current Documents and Processes
The first step is to identify where all your documents reside and how you are currently managing and organizing those documents. Additionally, an assessment of the documents themselves should be conducted to evaluate if they are still current, if they are in line with the processes and procedures they are intended to monitor, and if they are collecting all the required information.
Where are documents stored? What is electronic vs. paper? Are documents sorted by necessity, date, version, compliance area? What processes are currently in place for creating, managing, and storing documents? Where are the inefficiencies in adequately managing documents and records? If there are multiple systems, are they working together?
The goal of this step is to get a good handle on the current state of your documents and systems so you can move onto step 2, which will be to define the desired state of your document management system.
Step 2. Define Document Management System
Before building the system, you must define your ultimate desired end state. In a perfect world, how would the document management system operate? What parts and components would it have? How would things work together? At this point, you must consider the immediate need (i.e., document management) within the context of the overall business need. The idea is to align the document management system with any overall compliance management system (CMS). This requires a genuine understanding of both daily routines and the big picture.
Bring together key stakeholders to discuss their objectives, review the current state, and evaluate industry best practices. While it is necessary to get senior management buy-in and to understand the business needs, it is equally important to understand the routine activities and tasks of the people who will use the system in a daily basis. The system must be designed with all these users in mind—the end user entering data in the field, management who is reading reports and metrics, system administrator, office staff, etc.
Step 3. Gather Documents and Populate System
This step can involve significant resources depending on the volume of documents, so taking a phased approach can make it more manageable. It often makes sense to start where you already have processes and document storage systems in place that can be more easily transitioned into a new document management system to encourage user buy-in. Priorities should be set based on ease of implementation, compliance risk, business improvement, and value to the company.
Step 4. Determine Access and Train
The only way to ensure employees will correctly use the document management system is to provide adequate training. Define who needs access to the various parts of the system and what everyone’s roles and responsibilities are. Every employee who will touch the system should receive hands-on training to teach them how to correctly use the system to create efficiencies.
Step 5. Conduct an Annual Internal Audit and Document Review
Audits offer a systematic, objective tool to assess compliance across the workplace and to identify any opportunities for improvement. Audits may be used to capture regulatory compliance status, certification system conformance, adequacy of internal controls, potential risks, and best practices.
An internal audit of the document management system provides a valuable way to communicate performance to decision-makers and key stakeholders. This final step is an important one, because it will help ensure that:
- The organization is getting the most out of its document management system.
- The system and associated processes are operating as intended.
- Data can be used for trending and predictive analytics to better inform business decision-making.
- Ongoing opportunities for improvement in document organization and processes are identified and implemented.
- Efficiencies in business operations and overall compliance management—including remote access and remote auditing—are fully realized.

Validation vs. Verification: What’s the Difference?
To ensure a sound Hazard Analysis and Critical Controls Points (HACCP) Plan, companies must confirm the Plan is adequate for controlling food safety hazards through the process of validation and verification.
According to 9 CFR 417.4 a, “Every establishment shall validate the HACCP Plan’s adequacy in controlling the food safety hazards identified during the hazard analysis and shall verify that the Plan is being effectively implemented.” HACCP Principle 6—Establish Verification Procedures—further emphasizes the importance of establishing activities that determine the validity of the HACCP Plan and verify that the system is operating according to the Plan.
Based on these requirements, verification and validation seem quite similar. In practice, however, verification and validation are distinct functions that are both critical for compliance with U.S. Department of Agriculture (USDA) and Food and Drug Administration (FDA) regulations. In short, verification is focused on the implementation of the plan, while validation is focused on its accuracy. You cannot validate a process until you verify the process is consistently following the plan and operating as intended.
Validation: Proof the Plan Is Effective
Validation demonstrates and documents that the HACCP system works to address significant hazards. It provides proof that the Plan is effective. The purpose of validation is to demonstrate that the HACCP system, as designed, will adequately control identified hazards to produce a safe, unadulterated product. Following completion of the hazard analysis and development of the HACCP Plan, establishments enter the 90-day period of initial validation, where the validity of the HACCP system is checked. Is the Plan working to achieve its intended goal?
Validation involves gathering data over time to confirm something is operating as intended. It relies heavily on using scientific data from journals; in-plant observations, measurements, and evaluations; and expert advice. According to the National Advisory Committee on Microbiological Criteria for Foods (NACMCF), “Validation is the element of verification that focuses on collecting and evaluating scientific and technical information to determine if the HACCP Plan, when properly implemented, will effectively control the identified hazards.”
For example, validation of Critical Control Points (CCPs) may involve reviewing trends over the year, customer complaints, equipment issues, etc. to determine whether the process is working. To validate a temperature selected for heating food to remove harmful bacteria, a facility may cite scientific journals and studies.
Both USDA and FDA require validation of the food safety system to document scientific support for CCP or process preventive control critical limits. USDA further requires internal validation of the CCPs and critical operational parameters used in key prerequisite programs (PRPs). It is important companies use scientific evidence (e.g., microbiological test results, validation studies) to the extent possible to demonstrate hazards are effectively controlled.
Verification: Proof the Plan Is Followed as Written
Verification establishes the accuracy or truth of something—in other words, proof that the HACCP Plan is being followed as written. It answers the question, “Are we actually doing what we say we are going to do?” For example, if the Plan says that a food will be heated to a certain temperature to kill harmful bacteria, verification will test that the food actually reaches that temperature.
The purpose of verification is to confirm that the HACCP system is continually functioning as intended. Following the 90-day period of initial validation, monitoring and verification activities are performed to ensure the HACCP system continues to be implemented properly. These activities should be scheduled as needed (i.e., daily, weekly, monthly, quarterly, annually) and conducted by designated, trained employees.
Regular audits of the HACCP Plan further ensure that it is being followed correctly. This is particularly important if any aspect of the company’s procedure, process, or ingredients has changed or a new product has been added to production.
HACCP Principle 6 outlines four elements for verification:
- CCP Verification
- Overall Food Safety System Verification
- Food Safety System Validation
- Regulatory Verification
In addition, both USDA and FDA require verification of the overall food safety system. USDA requires reassessments to be performed annually to verify the HACCP Plan. FDA requires reanalysis to be performed at least every three years to verify the Food Safety Plan.
There are some common verification activities to ensure food manufacturing facilities meet these requirements:
- Document review, including HACCP Plan and related policies, plans, Good Manufacturing Practices (GMPs), standard operating procedures (SOPs), equipment and product specifications, processing rates, inspection records, supplier information, etc.
- Facility walk-through to review operations and observe specific processes and equipment, as needed
- Evaluation of current Food Safety Management System (FSMS) elements
- Food Safety Plan review
- Review of PRPs (e.g., sanitation, allergen controls, traceability)
- Environmental monitoring and product testing
- Confirmation that the CCPs and other preventive controls are implemented and effective
- Direct observations of CCP monitoring activities
- Calibration of equipment
Validation and verification are important components of any food safety system. They provide proof that the HACCP Plan is not only effective, but also being followed and working as intended. Validation and verification ensure the Plan is a living, breathing document that is used daily to ensure the food safety system complies with both USDA and FDA regulations and, more importantly, works to prevents foodborne illness.

Comments: No Comments
SQF V9: Planning for Changes
The Safe Quality Food (SQF) Program is a rigorous food safety and quality program. Recognized by the Global Food Safety Initiative (GFSI), the SQF codes are designed to meet industry, customer, and regulatory requirements for all sectors of the food supply chain. SQF certification showcases certified sites’ commitment to a culture of food safety and operational excellence in food safety management.
In May 2021, SQF will be releasing Edition 9 (SQF V9) to align the code with the latest GFSI benchmarking criteria, updated regulatory requirements, and scientific changes. According to the SQF Institute, SQF V9 is designed to help certified sites meet and exceed all industry, customer, and regulatory requirements so they can remain competitive across sectors. V9 is scheduled for implementation with audits beginning May 24, 2021.
Significant Changes
The SQF V9 changes are broken down into two categories of changes:
Structural Changes
- Development of Custom Codes for certain industry-specific sectors (livestock, animal feed, petfood, aquaculture, dietary supplements)
- Streamlined technical elements to reduce redundancy in the following sections:
- Site location and operation
- Food safety culture
- Chemical storage
- Personal hygiene
- Specifications
- Training
Technical Changes
- GFSI benchmarking requirement updates, including changes to:
- Food safety culture requirements
- Internal laboratory requirements
- HACCP plan requirements for storage and distribution
- Remote activities requirements
- SQF stakeholder feedback updates, including changes to:
- Co-manufacturers’ requirements
- Ambient air testing requirement
- Audit scoring
New or updated concepts that present some of the greatest changes under SQF V9 include the following:
- Food Safety Culture Requirements: Senior leadership is required to lead and support a food safety culture within the site.
- Additional Training Requirements: Training requirements are now defined for sampling and test methods, environmental monitoring, allergen management, food defense, and food fraud for all relevant staff.
- Labeling Requirements: Updates to Product Identification Section now emphasize labeling requirements and checks during operations and require the implementation of procedures to ensure label use is reconciled.
- Substitute SQF Practitioner: All sites are now required to have a designated substitute SQF Practitioner with HACCP training and competencies in maintaining the food safety plan and knowledge of the SQF Food Safety Code.
Planning for Change
For companies that are SQF-certified, now is the ideal time to assess current SQF program elements, identify improvements that are internally desirable and required by the new standard, and implement those updates that will make the SQF program more useful to the business. This can be done through a series of phases to ensure adoption throughout the organization.
Phase 1: SQF Assessment
An assessment should begin by reviewing the following:
- Existing SQF programs, processes, and procedures
- Existing document management systems
- Employee training tools and programs
This documentation review and program assessment will help to identify elements of the existing SQF program that are acceptable, those that show opportunities for improvement, and those that may be missing, including those needed for development and implementation to meet the requirements of SQF V9.
Phase 2: SQF Program Updates
The assessment will inform a plan for updating the SQF certification program, including major activities, key milestones, and expected outcomes. Development/update activities included on the plan may include the following:
- Updating current SQF programs, processes, and procedures with missing V9 requirements
- Developing new SQF programs, processes, and procedures for additional V9 requirements
- Updating training programs with any new and additional requirements
- Revising document register to align with SQF V9 numbering changes
- Updating records and forms with any new and additional requirements
- Updating Food Safety Policy to include new food safety culture requirements
When implementing program updates, leveraging existing management system and certification program elements and utilizing proven approaches can greatly streamline the process.
Phase 3: Training
To ensure staff are prepared to implement and sustain the updated SQF V9 program, training is important. This includes training for affected staff on applicable requirements; specific plans, procedures, and GMPs developed to achieve compliance; and the certification roadmap to prepare for future audits.
Following this plan now will help companies ensure they maintain their SQF certification when audits begin under SQF V9 in May 2021–and that certification matters when it comes to meeting customer and regulatory requirements, protecting the company brand, and keeping consumers safe.

Comments: 1 Comment
FDA’s New Era of Smarter Food Safety
The food system is rapidly evolving—from new foods, to new formulations, to new production and delivery methods. As a whole, the industry is pushing into untapped areas, facing supply chain challenges, and responding to unique market demands, including those that have quickly emerged as a result of the COVID-19 pandemic (e.g., e-commerce, new delivery models, virtual inspections).
To keep pace with all this change, the Food & Drug Administration (FDA) announced the New Era of Smarter Food Safety in April 2019. On July 13, 2020 (delayed from Spring 2020 due to COVID-19-related issues), the Administration subsequently published the New Era of Smarter Food Safety Blueprint. This blueprint outlines the approach FDA will take create a New Era of Smarter Food Safety that evolves along with food technologies and systems—and the impacts these changes will have on the food industry and consumers alike.
New Era: What to Expect
According to FDA, “The New Era of Smarter Food Safety represents a new approach to food safety, leveraging technology and other tools to create a safer and more digital, traceable food system.” That being said, the Administration further notes that “smarter food safety is about more than just technology. It’s also about simpler, more effective, and modern approaches and processes. It’s about leadership, creativity, and culture.”
Correspondingly, the New Era is built on four core elements to support FDA’s ultimate goal of reducing foodborne illness:
- Tech-Enabled Traceability
- Smarter Tools and Approaches for Prevention and Outbreak Response
- New Business Models and Retail Modernization
- Food Safety Culture
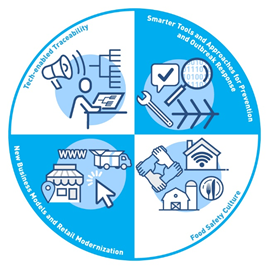
Importantly, the New Era does not replace or negate the progress of the Food Safety Modernization Act (FSMA). Rather it builds on FSMA’s science and risk-based protections and uses them as the foundation for integrating more data, better analytics, and technological advancements going forward. FSMA’s full implementation remains a priority for the FDA. In FDA’s words, the New Era is “people-led, FSMA-based, and technology-enabled”.
Building the Blueprint
The New Era of Smarter Food Safety Blueprint is the roadmap FDA will follow to build its New Era and further food safety modernization under the FSMA rules. The blueprint includes goals to:
- Enhance traceability
- Improve predictive analytics
- Respond more rapidly to outbreaks
- Address new business models
- Reduce contamination of food
- Foster the development of stronger food safety cultures
Each of these goals is addressed by the core elements and the following actions, as outlined in the blueprint, which will be implemented over the next decade:
Core Element 1: Tech-enabled Traceability. Food traceability is the ability to track any food through all stages of the supply chain—production, processing, distribution—to ensure food safety and operational efficiency. The objective of Tech-enabled Traceability is to use technology to create traceability advancements, including implementing rapid tracebacks, identifying specific sources, and helping to quickly remove products from the marketplace when necessary. Beyond technology, part of this effort involves harmonizing efforts to follow food from farm to table by creating similar data standards across government and industry. As public health agencies increasingly rely on electronic data in outbreak investigations, quality and compatibility must be addressed to more quickly and accurately trace the origin of contaminated food. Blueprint actions include:
- Developing foundational components
- Encouraging and incentivizing industry adoption of new technologies
- Leveraging the digital transformation
Core Element 2: Smarter Tools and Approaches for Prevention and Outbreak Response. One of the most powerful resources available to create this New Era is data. However, that power lies in collecting better quality data, conducting more meaningful analysis, and transforming data into more strategic, prevention-oriented actions. The blueprint seeks to strengthen procedures and protocols for conducting root cause analyses to identify how a food became contaminated and then for using predictive analytics to prevent future outbreaks. As under FSMA, the focus is on the preventive nature of modern food safety approaches. Blueprint actions include:
- Invigorating root cause analysis
- Strengthening predictive analytics capabilities
- Developing domestic mutual reliance
- Developing inspection, training, and compliance tools
- Improving outbreak response
- Implementing recall modernization
Core Element 3: New Business Models and Retail Food Modernization. How food is getting from farm to table is continually evolving. The recent pandemic is a prime example, as it has brought huge growth in distribution channels, including e-commerce and food delivery, carryout, and pickup. The FDA and industry must be prepared for new business models that continue to emerge with marketplace demands and consumer needs. The address this, blueprint actions include:
- Ensuring safety of food produced or delivered using new business models
- Modernizing traditional retail food safety approaches
Core Element 4: Food Safety Culture. According to the Safe Food Alliance, “Food safety culture refers to the specific culture of a facility: the attitudes, beliefs, practices, and values that determine what is happening when no one is watching. A strong culture of food safety helps a facility both to prevent and catch deviations in their processes that impact the safety, quality, and legality of their products.” Improvements in food safety, foodborne illness, and outbreaks depend largely on food safety culture, even more than technology. A strong food safety culture has always been a prerequisite to effective food safety management and that will continue in the New Era. Blueprint actions include:
- Promoting food safety culture throughout the food system
- Further promoting food safety culture throughout the Agency
- Developing and promoting a Smarter Food Safety consumer education campaign
Incorporating into Operations
As FDA has recognized, there is a real need for more “real-time, data-driven, nimble approaches to help ensure a strong and resilient food system”. The New Era of Smarter Food Safety Blueprint is FDA’s way to get there. It will be up to players in the food industry to take the core elements of the blueprint and begin to incorporate them into operations. As FDA rolls out this initiative, organizations should consider doing the following:
- Implement an information management system to help coordinate, organize, control, analyze, and visualize the information necessary to remain in compliance and operate efficiently.
- Conduct third-party assessments to provide an outside perspective of food safety systems and compliance/certification.
- Explore technological advancements that allow for further digitization and promote more timely and accurate collection and management of important data.
- Conduct root cause analysis, as needed, to identify underlying issues and ensure similar problems do not occur in the future.
- Build a strong food safety culture that focuses on changing from a reactionary to a preventive mindset that promotes safety and quality.

Comments: No Comments
Maintaining Food Safety Compliance: Third-Party Assessments
Regulatory, customer, and industry standards require that Food Safety Management Systems (FSMS) and programs must be current at all times. They also require that any changes be verified and validated. Third-party assessments provide a means of confirming compliance specific to key programs, particularly when many certification audits are being postponed or limited in scope due to COVID concerns.
Focused Activity, Desired Results
As a focused activity, third-party assessments support food safety compliance and certification and respond directly to a company’s needs:
- Third-party assessments provide more direct coverage to evaluate specific program effectiveness and allow for a more focused understanding of existing strengths and improvement areas.
- Enlisting a qualified outside firm further provides an unbiased assessment, offers an opportunity to work with local resources, and allows for a more flexible timeframe.
- The assessment typically results in a report developed by a qualified source focused on closing corrective actions.
- It minimizes the overall disruption in business compared to a full certification-level audit.
Major Program Assessments
While third-party assessments are not uncommon in the food industry, they are generally not interwoven with industry certification audits. That being said, an FSMS is comprised of major programs or sections that are prime subjects for third-party assessment, including the following.
A Hazard Analysis and Risk-Based Preventive Controls (HARPC) third-party assessment is based on a customized workplan, as well as the updated HARPC/Preventive Controls for Human or Animal Food rule. Aligning the assessment with internal audit programs supports verification by providing direct recommendations for corrections and improvements. This approach provides the following benefits:
- Assessment can be completed and delivered more directly with the Food Safety Team.
- Recommendations from findings can be more directly implemented and issues can be closed.
- Results can be verified and validated internally, providing a record of corrections to be implemented, meeting regulatory requirements, and demonstrating alignment with the internal audit program.
- The assessment provides a means to confirm timing of program updates and to determine the next scheduled assessment.
Current Good Manufacturing Practices (cGMP) compliance extends to FDA Section 117 requirements, which must be maintained under FSMA as part of the Food Safety Plan. A gap assessment of existing cGMPs confirms programs are complete and current, provides verification of updates, and validates programs are documented. Independent verification can be scheduled on a more flexible timeframe using qualified resources based on preference. Focused assessments of cGMPs provide a fresh look at programs, improvement recommendations, and direct corrective actions that are in line with the company’s internal audit processes.
Building and equipment assessments support site commissioning requirements for food and handling. Typically, building elements are not a major focus; however, assessing building and equipment is imperative, as it provides for opportunities to correct risks not always identified by the audit:
- Drills down to maintenance practices and provides a clear focus on preventive maintenance.
- Provides a perspective on physical plant processes that allows for planned improvements and hygienic design implementations.
- Ensures the integrity of physical food plant and site asset conditions.
Supplier program assessment provides a stronger level review of the organization’s suppliers. This assessment provides an opportunity to update supplier criteria and ratings and bring a pragmatic perspective for change based on qualified vs. underperforming suppliers. A clear benefit of the assessment is the ability to highlight established supplier performance in a more detailed way than is typically included in an audit to generate possible improvements.
Training should be an established food program and cGMP from the initial implementation of an FSMS. In the past, training was looked at as more casual than formal. As requirements have progressed, training has become more challenging. A focused assessment determines effectiveness and consistency of training by:
- Drilling down to job level
- Identifying alternatives to make training more effective
- Incorporating a broader level of industry observations
- Providing for planned training processes and program improvements
- Addressing culture and language barriers
Recently, KTL Principal Bill Bremer and Thomas Paraboschi, DNV GL’s Supply Chain and Digital Assurance Services Manager, joined International Food Safety & Quality Network (IFSQN) for a Food Safety Friday Webinar to discuss the benefits of increasing safety and quality through non-certification assessments. Join the replay of their conversation and learn more about this topic.

Environment / Food Safety / Quality / Safety / Technology Enabled Business Solutions
Comments: No Comments
Functionality for Today…Flexibility for the Future
There is no question about it—organizations across nearly every industry are relying more heavily on information technology (IT) to carry out daily tasks, connect staff, and manage operations. Technology can also play a vital role in managing compliance requirements.
For example, we recently shared a case study demonstrating how leveraging a simple Microsoft SharePoint®-based Compliance Management System (CMS) has provided Southeast Missouri State University (SEMO) with access to the data, documents, systems, and processes required to help employees effectively manage compliance requirements—even when working remotely.
Tips to Design a Successful CMS
A CMS is used to coordinate, organize, control, analyze, and visualize information to help organizations remain in compliance and operate efficiently. When building a CMS, it is important to follow a process to design a system that provides the functionality to meet current requirements and the flexibility to anticipate future needs.
The following eight tips can help ensure you end up with the right CMS and efficiency tools to support your organization for the long term:
- Inventory your existing systems – Identify how you are currently managing your compliance needs/requirements. What’s working well? What isn’t working? Do the systems work together? Do they all operate independently? This inventory should evaluate the following:
- Current systems and tools
- Status and functionality of existing processes
- Data sources and ability to pull information from various sources
- Organizational complexity
- Compliance status
- Existing management systems
- Determine your business drivers – Are you looking to save time? Create efficiencies? Provide access to enable employees to work from home? Reduce the number of resources required? Have better access to real-time information? Answer to senior management? Respond to regulatory requirements? These drivers will also drive the decisions you make when it comes to module development, dashboard design, reporting, and more.
- Understand the daily routine of the individuals using the system – Systems and modules should be built according to existing daily routines, when possible, and then implemented and rolled out in a way that encourages adoption. Having a solid understanding of routine tasks and activities will ensure the system is built in a way that works for the individuals using it—and for the way they will be accessing it.
- Understand your compliance requirements – Do you have permitting requirements? Does your staff need training? How do you maintain your records? Are there regular (e.g., annual, semi-annual) plans and/or reports you need to submit? Do you have routine inspections and monitoring? All these things can and should be built into a CMS so they can be managed more efficiently.
- Get the right parties involved – There are many people that touch a CMS at various points in the process. The system must be designed with all these users in mind: the end user entering data in the field, management who is reading reports and metrics, system administrator, office staff, etc. A truly user-friendly system will be something that meets the needs of all parties. If employees are frustrated by lack of understanding, if the system isn’t intuitive enough, if it is hard to put data in or get metrics out, the system will hold little value.
- Make your wish list – While you may start your project one module at a time, it is important to define your ultimate desired end state. In a perfect world, how would the CMS operate? What parts and components would it have? How would things work together? What type of interfaces would users have? You may build piece by piece, but you must develop with the end in mind.
- Set your priorities, budget, and pace – What is the most important item on your list? Do you want to develop modules one at a time or as a fully functional system? It often makes sense to start where you already have processes in place that can be more easily transitioned into a new system to encourage user buy-in. Priorities should be set based on ease of implementation, compliance risk, business improvement, and value to your company.
- Select the right consultant – For a CMS, it is valuable to have a consultant who doesn’t just understand technology but also understands your operational needs, regulatory obligations, and compliance issues. More than likely, off-the-shelf software will not be a silver bullet compliance solution. A consultant who can understand the bigger picture of where you want to go and will collaborate to design the right CMS and efficiency tools will bring the most value to your organization.
These tips can help ensure any organization designs and develops the right CMS—one that works within the organization’s operating environment—to reduce compliance risk, create efficiencies, provide operational flexibility, and generate business improvement and value.
