
Comments: No Comments
New Era of Smarter Food Safety: Two Years of Progress
Two years ago—on July 13, 2020—the Food & Drug Administration (FDA) published the New Era of Smarter Food Safety Blueprint, providing the roadmap FDA will follow to further food safety modernization under the Food Safety Modernization Act (FSMA). According to FDA, “Smarter food safety is about more than just technology. It’s also about simpler, more effective, and modern approaches and processes. It’s about leadership, creativity, and culture.”
This has remained the Administration’s focus as it has pushed its New Era of Food Safety forward to meet the Blueprint’s goals to:
- Enhance traceability
- Improve predictive analytics
- Respond more rapidly to outbreaks
- Address new business models
- Reduce contamination of food
- Foster the development of stronger food safety cultures
Progress to Date
For the past two years, FDA has focused on initiatives to support the four core elements identified in the Blueprint. Progress-to-date has included the following:
Core Element #1: Tech-Enabled Traceability. FDA has taken several actions to use technology to create food traceability advancements and reduce foodborne illness:
- The proposed Food Traceability Rule was published on September 23, 2020, as required under FSMA Section 204(d), to enhance traceability recordkeeping for certain identified foods beyond a limited “one-up, one-back” traceback approach. The final rule must be submitted by November 7, 2022.
- Twelve winning teams were selected in FDA’s Low- or No-Cost Tech-Enabled Traceability Challenge, which encouraged development of traceability systems that are cost-effective for food operations of all size. The winning entities are now working with FDA to disseminate their ideas to stakeholders.
Core Element # 2: Smart Tools for Outbreak Response. The Blueprint seeks to strengthen the use of data for root cause analyses and predictive analytics to prevent future outbreaks. Advancements in this area have included the following:
- FDA conducted a pilot study designed to strengthen the ability to predict which shipments of imported seafood pose the greatest risk of violation. Results show that machine learning could increase the likelihood of identifying a shipment containing potentially contaminated products.
- FDA has increased the amount and quality of data through information sharing agreements with regulatory and public health partners, academic institutions, industry, and others. This includes domestic mutual reliance agreements signed with five states (i.e., California, Florida, Minnesota, Utah, and Wisconsin), which “provide opportunities for the FDA and state partners to lay a quality foundation for sharing information and working together on regulatory services and food protection that industry and consumers can trust.”
- In December 2021, FDA released its Foodborne Outbreak Response Improvement Plan, which sets the stage to “enhance the speed, effectiveness, coordination, and communication of foodborne outbreak investigations” through tech-enabled product traceback, root cause investigations, stronger analysis and dissemination of outbreak data, and operational improvements.
Core Element #3: New Business Models and Retail Food Modernization. As FDA has acknowledged, “The COVID-19 pandemic underscores the need for modern approaches as we respond to unique demands on our food system, from unprecedented imbalances in the marketplace, to changing consumer behaviors and a rise in e-commerce, to challenges to performing inspection and compliance work in FDA’s traditional manner.” Correspondingly, the Administration is considering how to address potential food safety vulnerabilities for foods ordered online and delivered directly to the consumer.
Core Element #4: Food Safety Culture. The improvements in food safety, foodborne illness, and outbreaks outlined above depend largely on food safety culture. Every action the FDA takes is intended to help create an atmosphere where organizations are aware of and help to prevent any process and/or operational issues and deviations that may impact the safety and/or quality of their food products. FDA also remains focused on consumer education regarding safe handling of food.
A Look Ahead
As FDA continues to push its New Era of Food Safety forward and new challenges surface, it is important to understand the current landscape, set priorities, and commit the appropriate resources to ensure long-term sustainability. Certainly, the COVID-19 pandemic has heightened a number of issues and unique demands on the food safety system.
As the Blueprint enters year three, FDA will continue to advance its core elements and goals. Companies that operate in the food industry should consider undertaking the following activities to align with FDA’s objectives:
- Implement a food safety compliance management system to help coordinate, organize, control, analyze, and visualize the information necessary to remain in compliance and operate efficiently.
- Conduct third-party assessments to provide an outside perspective of food safety systems and compliance/certification to identify gaps in programs that need development/updates.
- Explore technological advancements that allow for further digitization and promote more timely and accurate collection and management of important data.
- Gather/manage data and conduct root cause analysis, as needed, to identify underlying issues and ensure similar problems do not occur in the future.
- Build a strong food safety culture that focuses on changing from a reactionary to a preventive mindset that promotes safety and quality.

Food Safety Summit Recap: Key Takeaways
The Food Safety Summit brings together the food safety community to learn more about today’s most crucial elements of food safety—from regulatory concerns and current industry trends to ongoing challenges and the latest technology and solutions.
This year’s Summit, held earlier in May, proved once again to be an engaging and informative meeting for all in attendance. Throughout the Summit, KTL’s food safety experts observed several common themes and challenges that the food industry is facing — challenges that your business may be encountering today.
We sat down with KTL’s attendees—Roberto Bellavia, Kasia Branny, Samantha Edwards, April Greene, and Joe Tell—to get their key takeaways from the Summit.
What topics were covered throughout the Summit?
The agenda for this year’s Summit was packed. Just some of the topics covered included cybersecurity, internal audits, food waste, food safety culture, foodborne illness, food safety management systems (FSMS), data-driven analytics, tech-enabled traceability, food recalls, microbial challenge studies, supply chain management, and sanitation.
Food safety culture headlined the agenda as the keynote topic this year, and it is clear this is an area garnering much more attention and visibility across the food industry. The keynote address provided the Food and Drug Administration’s (FDA) views on the importance of developing and nurturing food safety culture in the food industry, the industry perspective on implementing successful food safety culture strategies, and the importance of food safety culture through the eyes of the Department of Justice (DOJ).
KTL participated in a panel on virtual tools for food safety assessments. Panelists discussed how organizations are using remote technology to perform food safety assessments, food inspections, product and facility approvals, and similar tasks that are usually performed in person and onsite. The group discussed the practical, procedural, legal, and technology considerations any organization needs to develop efficient and effective remote audit protocols and maximize their potential use of remote tools.
In addition, KTL had a large turnout for our Solutions Stage presentation, Food Safety Management System Case Study: Using Microsoft 365® to Improve Compliance. KTL discussed how having a simple, centralized FSMS to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. The big secret: most companies already have the software they need in-house. An industry case study demonstrated a cost-effective approach for building an FSMS using the Microsoft 365 platform with SharePoint®.
What are the biggest challenges companies in the food industry are currently facing?
Not surprisingly, one of the biggest challenges we heard time and again is related to staffing, from turnover in the quality department to being understaffed in production. On a related note, many also noted challenges in finding qualified—and available—auditors. Also not surprising, budget constraints and shortages in the supply chain remain challenges to navigate. It is increasingly difficult to get raw materials for operations and finding truckers to get materials to/from facilities is hampering production.
There remains a lot of focus on the application of new FDA requirements—like Foreign Supplier Verification Program (FSVP) and Food Defense and Intentional Adulteration—and finding good systems to manage all the related documents. While this is not a new rule, supplier approval/verification programs, vulnerability assessments, and written food defense plans will remain a key focus as a surge in food demand and lack of supply has created an environment ripe for food fraud. It is likely that FDA intentional adulteration inspections could also ramp up.
Finally, many companies are experiencing other regulatory bodies (beyond FDA and USDA) taking much more interest in food companies. The Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA) have started to realize that they have been somewhat lax on inspecting these facilities. 2021 brought a significant uptick in EPA multi-media inspections, enforcement actions, and large penalties for violations, particularly related to anhydrous ammonia storage, risk management, and chemical accident prevention planning. Anhydrous ammonia is widely used as refrigerant in food facilities, including meat, poultry, and fish processing facilities; dairy and ice cream plants; wineries and breweries; fruit juice, vegetable juice, and soft drink processing facilities; cold storage warehouses; other food processing facilities; and seafood processing facilities aboard ships. Not only are companies trying to make do with fewer staff, but responsibilities are growing to include these environmental, health, and safety (EHS) regulatory compliance concerns, as well.
Are there any *new* food safety trends you heard about that companies should have on their radar?
Food safety culture. Food safety culture and its introduction into various certification schemes and regulations is a hot trend that will only grow in importance. Food safety culture is a core element of the FDA’s New Era of Smarter Food Safety. It is also a key component of the GFSI Benchmarking Requirements Version 2020 and, subsequently, is being integrated as a requirement into many of the benchmarked food safety certification standards. Best-in-class food safety cultures have robust systems in place to ensure consistent commitment, communication, procedures, training, performance measurement, and trust.
Cybersecurity. Cybersecurity is moving to the forefront for many. A new law was adopted that requires companies in listed industry sectors, including the food sector, to report to the Department of Homeland Security (DHS) within 72 hours of a cyberattack and within 24 hours if they paid ransom. Implementing regulations will clarify which companies are specifically subject to this reporting. Interestingly, Microsoft SharePoint was mentioned during the Summit as one of the safest technologies for storing and managing information compared to other food safety software from a cybersecurity perspective.
Rapid laboratory testing. Another interesting and very current discussion was about the new micronutrient and chemical analyses that have been developed to quickly test baby formula to help combat the baby formula shortage. There is a lot of opportunity and more “affordable” technology available to set up internal rapid laboratory testing to conduct product and environmental testing.
Data analytics. There is also a big push for usable data. Companies are starting to realize they need to have data they can use in a meaningful way to improve their systems. A well-designed and well-executed food safety program—with data trend analysis—provides an important tool for ensuring food safety. The goal should be to effectively capture and analyze audit data and then use that information to improve food safety and quality, achieve certification requirements, and enhance overall business performance.
Sustainable food management. The regulatory community has identified a real need for addressing food waste and the lack of circularity in the food industry. Wasted food makes up the largest percentage—over 20%—of any one material sent to landfills and incinerators each year in the U.S. The EPA, USDA, and FDA have already joined forces to address the magnitude of wasted food impacts across the U.S. through the U.S. Food Loss and Waste 2030 Champion program. Efforts to promote sustainable food management have begun extending to the state level and will continue.
Supplier expectations. Finally, companies (and customers) are setting higher expectations for the supplier and vendor companies they work with. As a result, more companies are pursuing certifications in areas they may not have previously considered to meet these customer expectations or, sometimes, to work globally. This can include anything from getting certified to various ISO management system standards (e.g., quality (ISO 9001), environment (ISO 14001), cybersecurity (ISO 27001), etc.), or participating in sustainability and corporate social responsibility reporting (e.g., Global Reporting Initiative (GRI), Carbon Disclosure Project (CDP), etc.).
What should companies in the food industry be doing now to plan for the future?
The shortage of workers—and resources, in general—is likely to continue for the foreseeable future. The days where it is typical to see someone at the same company for 25+ years are coming to a close! Managing the complexities of a FSMS and food safety program is challenging, even when fully staffed. Every regulatory agency and voluntary certification standard calls for companies to fulfill compliance requirements; supply chain and internal requirements create further complications and confusion.
One of the best things food companies can do to manage this challenge and plan for the future is to invest in going digital. Compliance efficiency and tracking tools are becoming essential to allow companies to do more with fewer resources. An integrated compliance management system brings various tools together to create one system that effectively manages compliance requirements, enables staff to carry out daily tasks and manage operations, and supports operational decision making by tracking and trending data that is collected daily by the team charged with implementation.

Benefits of an Integrated Management System
According to the International Organization for Standardization (ISO), are currently more than 80 Management System Standards (MSS)—80 different standards designed to help companies improve their performance across a diverse range of areas and sectors.
Most companies these days have some sort of management system, whether formal (e.g., ISO, Global Food Safety Initiative (GFSI)-benchmarked standard, industry-specific) or informal. And, because most companies have various aspects and functions to their operations, many actually may have more than one system to organize processes and business objectives.
While management systems by ISO’s definition are designed to “help organizations improve their performance by specifying repeatable steps that organizations consciously implement to achieve their goals and objectives,” having multiple systems to manage often overlapping requirements (i.e., regulatory, certification, supply chain, internal) can create redundancies, inefficiencies, extra work, and overall confusion.
Integrated Management Systems: The Basics
A management system is the organizing framework that enables companies to achieve and sustain their operational and business objectives through a process of continuous improvement (i.e., Plan-Do-Check-Act). It is designed to identify and manage risks through an organized set of policies, procedures, practices, and resources that guide the enterprise and its activities to maximize business value.
A management system should be a means to better align operational quality, safety, environment, food safety, security, energy, etc. with the business. An integrated management system does just this. It aligns an organization’s various systems and processes into one complete framework, enabling the organization to work as a single unit to implement specific best practices organization-wide, fulfill the requirements of multiple standards, and meet a unified set of business objectives.
Integration Business Benefits
Ultimately, the various MSS have many common points—and all work towards the goal of making the organization more effective and efficient. Developing an integrated management system allows organizations to align the standards, find common management system components (e.g., terminology, policies, objectives, processes, resources), and add measurable and recognizable business value, including the following:
Greater consistency. An integrated approach creates greater consistency across business facets when it comes to terminology, processes, procedures, expectations, etc., and, in turn, greatly improved focus on a common set of business objectives. With an integrated system, organizations can ensure that processes, methods, and practices are in place, documented, and consistently applied across the entire organization. A common documented framework such as this helps alleviate duplication of efforts, allows for a more complete view of the functional needs of the entire organization, and reduces variability in performance.
Optimized processes and resources. Integrated systems allow companies to optimize processes and resources and, subsequently, reduce the time it takes to do certain activities. Integrated management systems help organizations to maintain requirements and associated documents concurrently—particularly through use of an information system—streamlining the process and allowing the organization to focus on improvements rather than maintaining multiple systems. A common system enables better use of resources and better collaboration and communication across the company.
More strategic approach. Organizations can take a more strategic approach with an integrated management system because it focuses on managing all aspects of the business, not just one area. It provides clear methods and processes to identify and prioritize risks, set and monitor goals, communicate risks to employees and management, and allocate appropriate resources to mitigate them. It also establishes a common language among managers, executives, and employees, which enables better goal setting, priority ranking, and allocation of resources. As a bonus, integrated systems also make it much easier to implement an organization-wide information system capable of tracking and reporting on common activities and key performance metrics.
Forward-thinking. More and more organizations are expecting more from the companies they work with—and that includes management systems. The push for best practices over just regulatory compliance is a growing trend. Reliable and effective regulatory compliance is commonly an outcome of consistent implementation of a management system. Beyond that, an integrated management system allows organizations to more effectively manage those risks (i.e., compliance, financial, legal liability, brand reputation) that can significantly impact the entire supply chain.
Help from the Standards
Standards organizations such has ISO are making it as easy as possible to implement an integrated management system—whether formal or informal—because, plain and simple, it just makes business sense. For example, ISO has adopted a Harmonized Structure (formerly known as High-Level Structure) to make sure every ISO MSS is structured in the same way with ten universal sections. The ISO MSS also use Annex SL, which dictates how the MSS should be written and, again, is consistent across the various MSS. These efforts simplify use, streamline protocol, and encourage standardization across the ISO MSS.
Beyond that, ISO has published a Guide to Integrating Management System Standards (revised in 2018) to help organizations implement integrated management system design—ISO or not. According to Michael McLean, Convenor or the ISO working group that developed the handbook, “Many organizations benefit from multiple management systems to help them ensure their systems and processes are in line with their objectives and help them maintain their business model through ever-changing environments. This handbook provides a practical guide for organizations to effectively align their management systems with their strategies, plans, and operations.”
Taking the Next Steps
If you are operating with multiple management systems—or even if you have no management system at all—there are some basic steps to creating an integrated management system:
- Invest the time to understand the current scope of operations, functional departments, compliance requirements, governance structure, etc. across the entire organization as a whole, not just siloed departments.
- Conduct a gap assessment to evaluate the current (“as-is”) condition of any formal or informal management system(s) against the desired (“to-be”) condition (e.g., ISO, GFSI, industry-specific).
- Create a development and implementation plan outlining tasks and resources required to close any identified gaps and achieve those objectives.
- Determine key components of the integrated management system required to achieve business objectives.
- Identify common elements to be standardized and incorporated into an integrated system (e.g., policies, procedures, processes, metrics, training).
- Determine what information technology can support and streamline an integrated management system.
- Provide relevant training to all interested parties to truly operationalize the management system across the organization.
Whether formal or informal, integrated management systems provide organizations—both big and small, in any industry—a pillar for sustainable growth. By developing and implementing an aligned management system, organizations can achieve more consistent, reliable, and efficient performance across many areas, while adding measurable and recognizable business value.

Comments: No Comments
World Food Safety Day: Safer Food, Better Health
On June 7, countries around the globe will celebrate World Food Safety Day, focusing this year on “Safer food, better health” as the theme. Established by the United Nations General Assembly in 2018, World Food Safety Day is an annual observation intended to mobilize action to prevent, detect, and manage foodborne risks and improve human health. The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) of the United Nations jointly facilitate the observance of World Food Safety Day.
According to WHO, foodborne diseases affect 1 in 10 people worldwide each year. Safe food is a key contributor to reducing these foodborne illnesses and other poor health conditions, including impaired development, micronutrient deficiencies, noncommunicable and communicable diseases, and mental illnesses. Only when food is safe can we fully benefit from its nutritional value.
Everyone has a role to play in food safety—whether we grow, process, transport, store, sell, buy, prepare, or serve food. The way we build food systems and how we organize food supply chains can help achieve safer food for better health.
WHO is calling on everyone to join in World Food Safety Day to ensure safe food for all. Check out the Guide to World Food Safety Day to learn more about how various stakeholders can promote safer food and better health on World Food Safety Day this June 7 and every day.

Comments: No Comments
Doing Your Due Diligence: Food M&As
Any mergers & acquisitions (M&A) transaction, no matter the size or structure, can have a significant impact on the acquiring company—and food safety is a critical factor. As KTL reported in our Food Safety: Top Trends to Watch in 2022 article early this year, we are seeing more and more private equity firms investing in food companies and, subsequently, in evaluating their food safety infrastructure to determine the potential risk and overall value of the acquisition.
An assessment of the operations, production processes, equipment conditions, food safety management, quality, regulatory compliance, and all related documentation needs to be completed as part of any food-related acquisition. Prior to any acquisition, it is important to determine:
- Condition of operations (i.e., personnel, equipment, processes, facility) necessary to effectively meet existing performance standards.
- Level of food safety compliance to regulatory requirements and applicable voluntary industry certifications.
- Any potential high-level risks that would impact the transaction.
What to Expect
A food safety and quality assurance (FSQA) due diligence assessment should focus on a review of food safety and management practices, regulatory compliance, and overall level of implementation of FSQA processes to identify potential impacts to a planned investment. In general, it involves reviewing FSQA processes and procedures compared best practices in the industry, including:
- Compliance with Food and Drug Administration (FDA), U.S. Department of Agriculture (USDA), and other state and local regulations, including environmental, health, and safety (EHS).
- Programs and practices to manage Global Food Safety Initiative (GFSI) certifications (i.e., SQF, FSSC 22000, BRCGS, IFS), if applicable.
- Condition of operations (i.e., personnel, equipment, processes) necessary to effectively meet identified performance standards.
- Procedures and ability to ensure food safety during and after the transaction and any potential planned construction/moves.
- Capacity to appropriately and efficiently respond to customer complaints and the implementation of related corrective actions.
- Ability to effectively manage risks associated with the supply chain.
Due Diligence Approach
A typical FSQA due diligence assessment will include the following activities:
Review of documentation. All documents (e.g., policies, plans, standard operating procedures, forms, monitoring records, etc.) must be reviewed to identify potential gaps with applicable requirements and high-level FSQA risks. If construction and/or moving to a new facility is part of the acquisition, production plans, designs, and transition processes and plans for the new facility must also be reviewed to assess the level of effort and costs associated with getting the new plant operational.
Facility and programs implementation evaluation. An onsite assessment allows for facility tours, interviews with key staff, and “boots-on-the-ground” review of:
- Program elements for level of development, compliance with applicable regulatory requirements, implementation integrity, organizational resources, and management support and commitment.
- Operational processes and conditions related to the handling, storage, production, and distribution of products.
- Key building, equipment, process, and controls components, condition, and functionality as they relate to potential food safety or EHS risks.
- Disclosure and verification regarding the handling of ingredients, labeling, and packaging.
- Food safety and EHS regulatory compliance history and potential risks (e.g., recall programs and new FDA regulations implementation).
Written report. In most cases, the final deliverable will be a written report documenting assessment activities and results, including operational conditions, major FSQA risks, and review of facility plans. The written report should include actionable items that will allow the investor to make informed decisions regarding the acquisition, as well as potential future remediation and/or improvements to existing programs. Typical reports should include:
- Results of documents and records reviews, with trends analysis
- Identification of positive practices
- Regulatory compliance concerns and performance history
- Food safety and EHS risks
- Opinion on priority, transition, and feasibility of production startup timeline considering food safety requirements
- Recommendations for improvement
Smooth Transition
Undertaking adequate due diligence in any M&A activity is vital. It can lead to the discovery of regulatory inconsistencies that may lessen the value of an entire product line or business—and opportunities to make improvements. It can provide better insights into the risks and potential benefits of a transaction. And, ultimately, thorough due diligence can help ensure a smoother, more effective, and sustainable business integration.

Visit KTL at the 2022 Food Safety Summit
As one of the premier events in the food industry, the Food Safety Summit provides a comprehensive conference and expo for attendees to learn from subject matter experts, exchange ideas, and find solutions to current industry challenges. The Summit is back in person this year, and KTL is excited to be there live!
- When: May 9-12, 2022
- Where: Donald Stephens Convention Center, Rosemont, Illinois
- Who: Retailers, food processors, distributors, food manufacturers, growers, foodservice, testing laboratories, importing/exporting, law firms, and other food safety professionals
- Find KTL: Stop by our booth (#125) in the exhibit hall!
KTL Solutions Stage Presentation
Be sure to also update your agenda to attend KTL’s Solutions Stage presentation on Thursday, May 12 at 1:30 pm. KTL Principal Joe Tell and Partner and Senior Consultant Roberto Bellavia will be highlighting the following case study:
Food Safety Management System Case Study: Using Microsoft 365® to Improve Compliance
Food and beverage companies and their suppliers are subject to a wide range of complex regulatory and certification requirements, often with limited resources to maintain and demonstrate compliance. Finding effective information management tools is critical. Having a simple, centralized FSMS to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. The big secret: most companies already have the software they need in-house. KTL will present an industry case study demonstrating a cost-effective approach for building an FSMS using your existing Microsoft 365® platform with SharePoint®.

Comments: No Comments
FDA Issues Guidance for Voluntary Recalls
Foodborne illnesses impact millions of Americans every year—ranging from mild cases to hospitalizations to death. Reducing foodborne illness is a focus for the U.S. Food and Drug Administration (FDA), as reflected in several recent actions, including the proposed Food Traceability Rule (published on September 23, 2020) and the 2021 Foodborne Outbreak Response Improvement Plan (FORIP).
FDA recently took another step toward reducing the public’s exposure to the risks of foodborne illness on March 3, 2022, issuing its final guidance for voluntary recalls: Initiation of Voluntary Recalls Under 21 CFR Part 7, Subpart C.
About Voluntary Recalls
A voluntary recall involves actions taken by a company to correct or remove a violative product from the market, either on its own initiative or in response to a recommendation from the FDA. FDA Associate Commissioner of Regulatory Affairs Judith McMeekin, Pharm. D., emphasizes that “voluntary recalls continue to be the fastest, most effective way for a company to correct or remove violative and potentially harmful products from the market to help keep consumers safe.”
FDA’s new guidance outlines the steps companies should take before a recall is mandated, including developing policies and procedures, establishing training, maintaining records, and initiating communications.
BEFORE: Preparation Guidance
FDA’s guidance applies to any products that fall under the agency’s jurisdiction, including any food, drug, or device intended for human or animal use. The guidance breaks down the agency’s recommendations for activities companies (including manufacturers and distributors) should take in advance of a potential recall to be “recall ready.” These include the following:
- Establish a recall communications plan to address communications with employees, FDA, supply chain, direct accounts, and the public, as necessary. Identify key contacts and develop draft templates that can be easily customized and distributed when needed.
- Identify and train appropriate personnel (and alternates) on recall-related responsibilities. The recall team should have a thorough understanding of recall procedures and their respective roles in carrying out a recall plan. Regular training, including mock recalls, help ensure competency.
- Identify any reporting requirements for distributed products, if required by FDA. This might include a report to the Reportable Food Registry, an adverse event report for a dietary supplement, or a report to FDA upon product correction or removal.
- Establish and implement product coding to ensure traceability throughout production and distribution. This will enable more effective lot identification to accurately define and limit the recall scope.
- Maintain distribution records to allow for easier and faster identification of products to be recalled. Distribution records should include contact information for all direct accounts that received the product.
- Establish recall initiation procedures. Prepare, maintain, and document written procedures for initiating a recall to minimize delays and uncertainty when/if a voluntary recall becomes necessary. Procedures should be covered in training and should describe actions to carry out the following:
- Stop distribution, shipment, and/or sales of affected products.
- Outline a recall strategy that considers the scope/depth of the recall and associated risks.
- Notify direct accounts throughout the distribution chain and communicate instructions for appropriate disposition of product.
- Notify the public, when appropriate, if a product presents a health hazard.
DURING: Identifying and Initiating a Potential Recall
Identifying a potential problem is the first step in initiating a potential recall. It is vital that companies ensure timely identification and response to product problems that might lead to a recall. FDA suggests that the following indicators may suggest a potential concern:
- Internal reports of product specification deviation
- Out-of-specification testing results
- Consumer complaints
- Inspectional observations or laboratory results
- Reports of adverse events (e.g., illness, injury, death)
It is then the company’s responsibility to investigate the potential problem to determine 1.) whether a deviation has occurred, and 2.) whether the safety, effectiveness, purity, or potency of distributed products has been affected. A voluntary recall should not be delayed pending results of this investigation. Rather, companies should follow established procedures (see above) to decide whether to initiate a recall, determine the appropriate scope/depth of the recall, and resolve whether to discontinue production or distribution of impacted product(s).
To initiate a voluntary recall, companies should:
- Notify the FDA immediately if the company believes the product to be violative.
- Promptly issue recall communications to affected direct accounts and to the general public, if appropriate.
- Follow the procedures established in the company’s recall plan to implement the recall in accordance with 21 CFR 7.46.
- Provide instructions to the distribution chain regarding disposition of the product.
AFTER: Working with FDA
The FDA is committed to working with companies to help facilitate prompt response (i.e., removal or correction) to violative product that enters the marketplace. The agency has designated recall coordinators organized by product type located throughout the country who can work with company recall teams to develop strategies, review communications, monitor disposition of the product, coordinate with other regulatory bodies, etc.
The FDA may request a company initiate a recall under 21 CFR 7.45 after conducting discussions with the firm if the product presents a risk of illness, injury, or gross consumer deception; the firm has not initiated a voluntary recall; or agency action is required to protect public health.
Reducing Risks
The food industry and supply chains continue to change and evolve at an accelerated pace. That introduces new risks and challenges, including foodborne illness outbreaks. FDA’s most recent guidance is intended to help companies proactively address these risks—reducing response time in a foodborne illness outbreak and, subsequently, the number of people impacted.

Comments: No Comments
Controlling Combustible Dust: Focus on Food
There is a wide range of agricultural and food products that create fire and explosion risks and hazards, including flour, grain, sugar, spices, cereal, flavoring additives, and many more. Dusts produced in any industry present challenges, but when manufacturing and processing food products, dusts can create the following major threats:
- Airborne dust can cause serious harm to human (employee) health by causing physical ailments ranging from dermatitis to occupational asthma to lung cancer.
- Dust can create the potential for cross contamination and encourage the spread of pathogens and allergens within the food processing plant.
- Dust may become combustible and serve as the source for explosions that harm workers, damage machinery, and destroy buildings/corporate reputation.
About Combustible Dust
The food industry is responsible for the largest percentage of combustible dust incidents in the U.S.—ranging anywhere from 24-43% depending on the timeframe and the source. One of the most notable combustible dust incidents in history involved a secondary explosion at the Imperial Sugar refinery in 2008, which killed 14 people and injured 40.
According to the Occupational Safety and Health Administration’s (OSHA) definition, combustible dust is “a solid material composed of distinct particles or pieces, regardless of size, shape, or chemical composition, which presents a fire or deflagration hazard when suspended in air or some other oxidizing medium over a range of concentrations.”
A dust explosion occurs when all five elements of the Dust Explosion Pentagon are present:
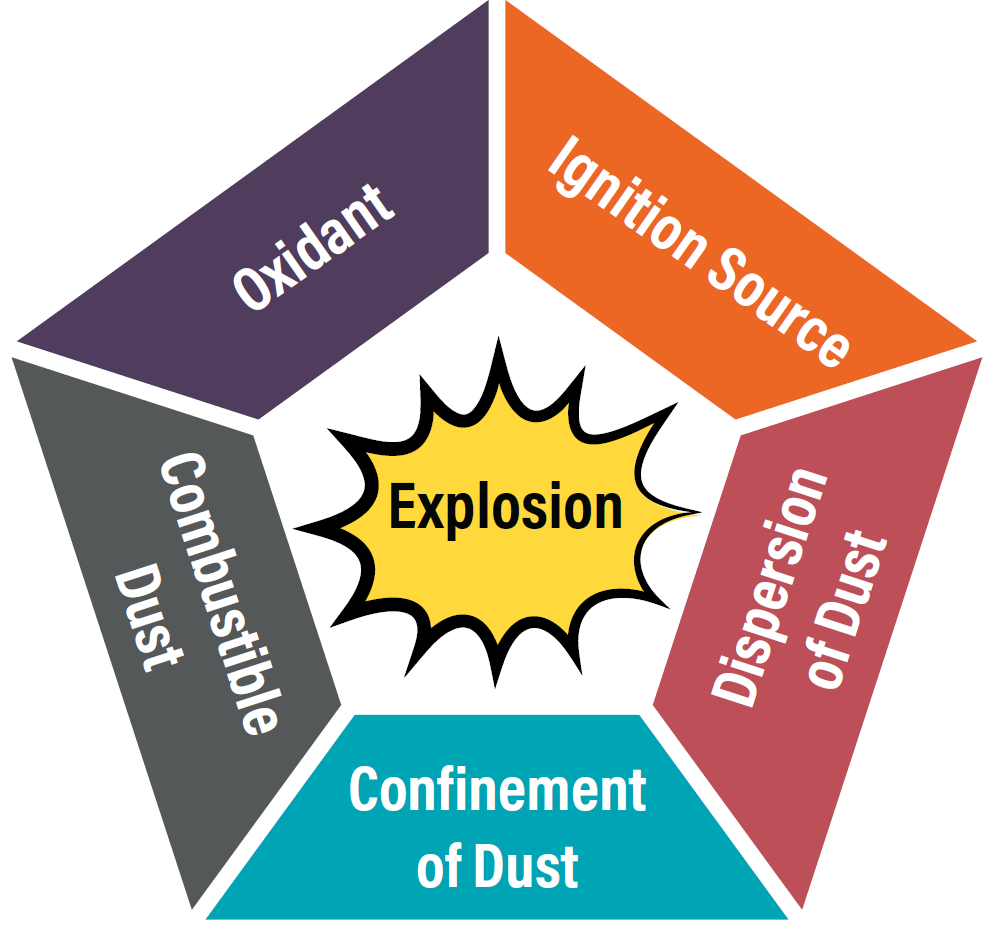
- Ignition source
- Oxygen
- Confined area
- Suspended cloud
- Explosive dust
Combustible Dust Standards
The devastating 2008 sugar refinery explosion prompted the development of OSHA’s Combustible Dust National Emphasis Program (NEP), and yet, some 14 years later, there is still no combustible dust regulation. That doesn’t mean, however, that there are no standards for managing and controlling combustible dust. In fact, there are several agencies involved in ensuring the food industry is appropriately managing the various hazards associated with combustible dust, including:
- National Fire Protection Association (NFPA)
- OSHA
- U.S. Food and Drug Administration (FDA)
NFPA Codes
NFPA sets standards regarding combustible dust. In fact, there are at least ten NFPA standards related to combustible dust (NFPA 61, 68, 69, 77, 484, 499, 652, 655, 664, 654). While these standards and codes are not law, per se, they are enforced by OSHA and referenced extensively. Most insurance agencies and local fire codes state that NFPA standards shall be followed as code.
NFPA’s most recent Standard on the Fundamentals of Combustible Dust (NFPA 652) covers the fundamental requirements for managing combustible dust fires and explosions and is an excellent starting point. NFPA 652 requires owners/operators to:
- Determine the dust’s combustibility and explosibility hazards based on laboratory testing or historical facility data.
- Conduct a Dust Hazard Analysis (DHA) to identify and evaluate potential dust fire and explosion hazards based on Kst (i.e., explosibility) values. Note: The DHA is a relatively new requirement—similar to OSHA’s Process Hazard Analysis as part of the Process Safety Management (PSM) program—used to assess risk and determine the required level of fire and explosion protection from combustible dust.
- Manage all the identified fire, flash fire, and explosion hazards identified in the DHA.
- Establish a written safety management system, including operating procedures and practices, training, incident investigation, and employee participation, to prevent and protect against the hazards.
NFPA 61 – Standard for the Prevention of Fires and Dust Explosions in Agricultural and Food Processing Facilities specifically covers facilities engaged in dry agricultural bulk materials or manufacturing and handling starch.
OSHA National Emphasis Program (NEP)
While OSHA has no formal standard for managing combustible dust, the agency published the Combustible Dust NEP in 2009, which outlines policies and procedures for inspecting workplaces that create or handle combustible dusts. The NEP heavily references the standards published by NFPA. It also references several other OSHA standards, including:
- 1910.22 Housekeeping
- 1910.307 Hazardous Locations
- 1910.1200 Hazard Communication
- 1910.269 Electric Power Generation, Transmission, and Distribution
- 1910.272 Grain Handling Facilities
- General Duty Clause, Section 5(a)(1) of the OSH Act
The General Duty Clause may be the most important of these, as it essentially gives OSHA the right to issue a citation any time employers fail to keep their employees safe from recognized hazards, including those hazards associated with combustible dust. Companies must control dust emissions to protect workers from exposure. If OSHA determines that even a very low Kst (i.e., low explosibility) dust is present in a facility with no explosion protection in place, the agency will issue citations and fines for lack of compliance.
Incidentally, OSHA published the Occupational Exposure to Flavoring Substances – Safety & Health Information Bulletin (SHIB) in 2010, cautioning that volatile organic compounds (VOCs) and respirable dust may be produced when handling many powdered flavoring formulations or spices. Inhalation of these substances may result in exposure directly to the small airways of the lungs.
FDA: Food Safety Modernization Act (FSMA)
In addition to meeting NFPA standards and OSHA guidelines, food companies must meet the requirements of FDA’s Food Safety Modernization Act (FSMA). FSMA requires food processing facilities to implement measures to ensure contamination hazards will be minimized or prevented. This includes developing a Hazard Analysis and Critical Control Points (HACCP) Plan with preventive controls for processes, food allergens, foreign objects, sanitation, and supply chain, as well as complying with Good Manufacturing Practices (GMPs).
Best Practices
Combustible dust itself is not avoidable, but the related consequences are. When it comes down to it, poor housekeeping is the biggest root cause of combustible dust issues. Many risks and hazards can be avoided by effectively cleaning the facility and equipment to remove contaminants before they become widely dispersed. Diligent housekeeping, combined with installing a dust collection system that is properly designed for the operation, can significantly reduce airborne dust and help mitigate a primary or more deadly secondary explosion.
If you work in the food industry, combustible dust is a concern. Make sure you understand the applicability of all the standards and codes, particularly the requirements under the various NFPA standards. The following may be required to keep your facility in compliance and your employees safe:
- Laboratory screening to determine if your powders are combustible or explosible.
- DHA to identify and evaluate potential dust fire, flash fire, and explosion hazards.
- Full analytical testing to determine explosion strength (Kst), minimum ignition energy, maximum safe storage temperature, and more.
- A written management system that documents operating procedures and practices for the ongoing management of dust fire, flash fire, and explosion hazards.
- Initial and ongoing training for operating, engineering, and management staff to build competency and understanding.

Comments: No Comments
Food Safety: Top Trends to Watch in 2022
The food system and supply chains continue to change and evolve at an accelerated pace. That introduces new risks and challenges—and heightens some familiar ones. As we move into 2022, the Food and Drug Administration (FDA) continues to strengthen its focus on the four core elements of its New Era of Smarter Food Safety to address several ongoing challenges in food safety. In addition to those identified in FDA’s New Era, KTL’s food safety experts have identified some of the key trends they are tracking in 2022—along with some guidance on how to prepare for the future.
Food Traceability
Food traceability is the ability to track any food through all stages of the supply chain—production, processing, distribution—to ensure food safety and operational efficiency. There is a real need for standardization, stronger linkages throughout the supply chain, improved communication and recordkeeping, and faster response. Notably, one of the core elements of FDA’s New Era is Tech-Enabled Traceability, which involves the use technology to create food traceability advancements. Under this, FDA has taken several actions to reduce foodborne illness:
- The proposed Food Traceability Rule was published on September 23, 2020, as required under the Food Safety Modernization Act (FSMA) Section 204(d), to enhance traceability recordkeeping for certain identified foods beyond a limited “one-up, one-back” traceback approach. The final rule must be submitted by November 7, 2022.
- FDA’s 2021 Foodborne Outbreak Response Improvement Plan sets the stage to “enhance the speed, effectiveness, coordination, and communication of foodborne outbreak investigations” through tech-enabled product traceback, root cause investigations, stronger analysis and dissemination of outbreak data, and operational improvements.
To prepare: Familiarize yourself with FDA’s proposed rule. Review the Food Traceability List (FTL) and start building systems and processes now that address requirements for traceability lot codes, critical tracking events (CTEs), key data elements (KDEs), and recordkeeping.
Food Safety Culture
An organization’s food safety culture is ultimately reflected in the way food safety is managed in the workplace. A strong food safety culture creates an atmosphere where everyone in the organization is aware of and helps to prevent any process and/or operational issues and deviations that may impact the safety and/or quality of their products. Food safety culture is another core element of the FDA’s New Era. It is also a key component of the GFSI Benchmarking Requirements Version 2020 and, subsequently, is being integrated as a requirement into many of the benchmarked food safety certification standards. Best-in-class food safety cultures have robust systems in place to ensure consistent commitment, communication, procedures, training, performance measurement, and trust.
To prepare: The incorporation of food safety should translate to all aspects of the business. Assess current food safety program elements, identify improvements that are internally desirable and required, and implement those updates that will create a strong food safety culture.
Sustainable Food Management
Wasted food makes up the largest percentage—over 20%—of any one material sent to landfills and incinerators each year in the U.S. With more customers and businesses focusing on making sustainable changes, food businesses need to work hard to ensure less food is being wasted. According to the Environmental Protection Agency (EPA), sustainable management of food is “a systematic approach that seeks to reduce wasted food and its associated impacts over the entire lifecycle, starting with the use of natural resources, manufacturing, sales, and consumption, and ending with decisions on recovery of final disposal.” The EPA, U.S. Department of Agriculture (USDA), and FDA joined forces to address the magnitude of wasted food impacts across the U.S. through the U.S. Food Loss and Waste 2030 Champion program. Efforts to promote sustainable food management have also extended to the state level.
To prepare: A thorough food and packaging assessment can help identify appropriate strategies to avoid waste, cut down on disposal costs, reduce over-purchasing and labor costs, reduce water and energy use associated with food production, and reduce GHG emissions. Based on the outcomes of the food waste assessment, there are some common strategies for reducing wasted food and packaging.
Health & Safety and Environmental Focus
During the pandemic, businesses had to find new ways of working to ensure health and safety. Employee protection will remain a top priority, particularly with potential increases in Occupational Safety and Health Administration (OSHA) funding to make improvements in workplace safety protection for American workers, as well as significant increases in OSHA’s maximum penalties. In addition, 2021 brought a significant uptick in EPA multi-media inspections, enforcement actions, and large penalties for violations, particularly related to anhydrous ammonia storage, risk management, and chemical accident prevention planning. Anhydrous ammonia is widely used as refrigerant in food facilities, including meat, poultry, and fish processing facilities; dairy and ice cream plants; wineries and breweries; fruit juice, vegetable juice, and soft drink processing facilities; cold storage warehouses; other food processing facilities; and seafood processing facilities aboard ships.
To prepare: Make sure you have the processes, programs, and systems in place—and documented—to ensure you are always protecting employees’ safety and health and meeting OSH Act requirements. If your facility uses anhydrous ammonia, you must understand the hazards posed by chemicals at the facility and design and maintain a safe facility to prevent accidental releases.
Compliance Efficiency Tools
Managing the complexities of a management system is challenging. That is because every regulatory agency (e.g., FDA, USDA, OSHA, EPA) and voluntary certification (e.g., GFSI-benchmarked standards, gluten-free, organic, ISO) calls for companies to fulfill compliance requirements—many of which overlap. Supply chain and internal requirements can create further complications and confusion. And more frequently than not, companies may not understand or have the resources to manage everything that needs to be in place to satisfy requirements. This is an area where compliance efficiency and tracking tools are becoming essential to allow companies to do more with fewer resources.
To prepare: An integrated compliance management system (CMS) is intended to bring various tools together to create one system that effectively manages compliance requirements, enables staff to carry out daily tasks and manage operations, and supports operational decision making by tracking and trending data that is collected daily by the team charged with implementation.
Food Fraud
In May 2016, FDA issued its final rule on Mitigation Strategies to Protect Food Against Intentional Adulteration (IA). While this is not a new rule, supplier approval/verification programs, vulnerability assessments, and written food defense plans will remain a key focus as a surge in food demand and lack of supply has created an environment ripe for food fraud. It is likely that FDA IA inspections could also ramp up, especially when more onsite inspections resume.
To prepare: Develop a food defense plan. This should include conducting a vulnerability assessment and developing the required programs to remove the risks of IA and food fraud.
Others to Watch…
- Food Irradiation. Food irradiation is a food safety technique used to kill pathogens, including bacteria, viruses, and parasites, and extend shelf life. Irradiation provides excellent safe food processing. FDA continues to research and approve irradiation for a variety of foods. Despite consumer resistance, expect this trend to continue.
- Food Investments. We are seeing more and more private equity firms investing in food companies and, subsequently, in their food safety infrastructure. Food safety is a critical factor in these acquisitions. Expect a thorough assessment of the condition of operations (i.e., personnel, equipment, processes, plant); level of food safety compliance/certification status; and any other potential risks that would impact the transaction.
- Cannabis. The regulatory framework for managing cannabis production is still murky. However, this is a rapidly growing market, and we anticipate progress—whether at the state level or federal level—in the development and implementation of regulations and controls (e.g., Good Manufacturing Practices (GMPs), labeling requirements, etc.).
- Labeling. COVID significantly increased the number of new food preparation and delivery/takeout services and ready-to-eat (RTE) food products. These new business models heighten the importance of appropriate labeling, particularly regarding allergens and instructions for safe food production.
Set Your Goals for 2022
As FDA continues to push its New Era of Food Safety forward and new challenges surface, it is important to understand the current landscape, set priorities, and commit the appropriate resources to ensure long-term sustainability. Consider undertaking the following activities:
- Implement a CMS to help coordinate, organize, control, analyze, and visualize the information necessary to remain in compliance and operate efficiently.
- Conduct third-party assessments to provide an outside perspective of food safety systems and compliance/certification to identify gaps in programs that need development/updates.
- Explore technological advancements that allow for further digitization and promote more timely and accurate collection and management of important data.
- Conduct root cause analysis, as needed, to identify underlying issues and ensure similar problems do not occur in the future.
- Build a strong food safety culture that focuses on changing from a reactionary to a preventive mindset that promotes safety and quality.
If you would like help evaluating your current food safety risk level and assessing your priorities for 2022, please contact KTL.

BioForward Member Blog: KTL Building Scalable Systems to Help Businesses
BioForward Wisconsin closely follows news stories about its members and invites them to contribute blogs and profiles to inform and advance the Wisconsin biohealth community. Read the recent BioForward Wisconsin member blog on KTL’s scalable systems for helping companies manage compliance business processes more efficiently and effectively.
