
Comments: No Comments
Doing Your Due Diligence: Food M&As
Any mergers & acquisitions (M&A) transaction, no matter the size or structure, can have a significant impact on the acquiring company—and food safety is a critical factor. As KTL reported in our Food Safety: Top Trends to Watch in 2022 article early this year, we are seeing more and more private equity firms investing in food companies and, subsequently, in evaluating their food safety infrastructure to determine the potential risk and overall value of the acquisition.
An assessment of the operations, production processes, equipment conditions, food safety management, quality, regulatory compliance, and all related documentation needs to be completed as part of any food-related acquisition. Prior to any acquisition, it is important to determine:
- Condition of operations (i.e., personnel, equipment, processes, facility) necessary to effectively meet existing performance standards.
- Level of food safety compliance to regulatory requirements and applicable voluntary industry certifications.
- Any potential high-level risks that would impact the transaction.
What to Expect
A food safety and quality assurance (FSQA) due diligence assessment should focus on a review of food safety and management practices, regulatory compliance, and overall level of implementation of FSQA processes to identify potential impacts to a planned investment. In general, it involves reviewing FSQA processes and procedures compared best practices in the industry, including:
- Compliance with Food and Drug Administration (FDA), U.S. Department of Agriculture (USDA), and other state and local regulations, including environmental, health, and safety (EHS).
- Programs and practices to manage Global Food Safety Initiative (GFSI) certifications (i.e., SQF, FSSC 22000, BRCGS, IFS), if applicable.
- Condition of operations (i.e., personnel, equipment, processes) necessary to effectively meet identified performance standards.
- Procedures and ability to ensure food safety during and after the transaction and any potential planned construction/moves.
- Capacity to appropriately and efficiently respond to customer complaints and the implementation of related corrective actions.
- Ability to effectively manage risks associated with the supply chain.
Due Diligence Approach
A typical FSQA due diligence assessment will include the following activities:
Review of documentation. All documents (e.g., policies, plans, standard operating procedures, forms, monitoring records, etc.) must be reviewed to identify potential gaps with applicable requirements and high-level FSQA risks. If construction and/or moving to a new facility is part of the acquisition, production plans, designs, and transition processes and plans for the new facility must also be reviewed to assess the level of effort and costs associated with getting the new plant operational.
Facility and programs implementation evaluation. An onsite assessment allows for facility tours, interviews with key staff, and “boots-on-the-ground” review of:
- Program elements for level of development, compliance with applicable regulatory requirements, implementation integrity, organizational resources, and management support and commitment.
- Operational processes and conditions related to the handling, storage, production, and distribution of products.
- Key building, equipment, process, and controls components, condition, and functionality as they relate to potential food safety or EHS risks.
- Disclosure and verification regarding the handling of ingredients, labeling, and packaging.
- Food safety and EHS regulatory compliance history and potential risks (e.g., recall programs and new FDA regulations implementation).
Written report. In most cases, the final deliverable will be a written report documenting assessment activities and results, including operational conditions, major FSQA risks, and review of facility plans. The written report should include actionable items that will allow the investor to make informed decisions regarding the acquisition, as well as potential future remediation and/or improvements to existing programs. Typical reports should include:
- Results of documents and records reviews, with trends analysis
- Identification of positive practices
- Regulatory compliance concerns and performance history
- Food safety and EHS risks
- Opinion on priority, transition, and feasibility of production startup timeline considering food safety requirements
- Recommendations for improvement
Smooth Transition
Undertaking adequate due diligence in any M&A activity is vital. It can lead to the discovery of regulatory inconsistencies that may lessen the value of an entire product line or business—and opportunities to make improvements. It can provide better insights into the risks and potential benefits of a transaction. And, ultimately, thorough due diligence can help ensure a smoother, more effective, and sustainable business integration.

Comments: No Comments
Staff Spotlight on Lisa Langdon, PE
Get to know our KTL team! This month, we are catching up with KTL Principal Lisa Langdon, P.E. Lisa provides project direction and strategic oversight to ensure the success of KTL’s projects and satisfaction of our clients. She has over 25 years of experience assisting clients with management system design and deployment, business process improvement, risk management, and compliance assurance. Lisa also manages KTL’s team of consultants and support staff. She is based out of our Madison, WI headquarters.
Tell us a little bit about your background—what are your areas of expertise?
I have my degree in Civil Engineering with an environmental emphasis from the University of Wisconsin-Madison. I began my career in California, primarily doing solid waste engineering work. During that time, I became a registered Professional Engineer.
With my Midwestern roots, we moved back to the Madison area, and I began working at what was then known as Kestrel Management Services (now KTL). I wanted to transition into helping companies manage their environmental risks—beyond just remediating their environmental compliance issues. This has been the focus of my career for the past 20 years at KTL. I work primarily on management system design and deployment, business process improvement, risk management, and compliance assurance. In particular, my areas of expertise include analyzing EHS and other business processes and identifying and implementing improvement opportunities to ensure compliance and enterprise risk reduction.
In 2014, I took partial ownership of KTL and am now currently a principal owner and member of KTL’s Executive Committee. In this role, I continue to provide executive-level project direction and oversight, manage client relationships to effectively meet needs, oversee internal processes and systems, and manage KTL’s technical operations and staff.
What types of clients do you work with? What are the biggest issues you see them facing right now?
I have worked for a wide variety of companies ranging from a Class I railroad, to utilities and chemical companies, to municipal departments. While the majority of our projects involve solving issues and looking for opportunities to improve EHS and food safety compliance, we can help provide process improvement and risk reduction services to any type of organization. As a very recent example, we will use our systems analysis approach to work on a government-funded project in cooperation with pharmacy chains to develop strategies to improve access to medications for Opioid Use Disorder (OUD). While the industry and compliance issues are different, KTL’s overall approach on this project remains the same.
As for the biggest issues I see right now, I think it is safe to say that many of our clients continue to struggle with finding and retaining quality staff. KTL continues to help many companies fill this hole by supporting their EHS or food safety departments to ensure ongoing compliance requirements are met. We also see many companies still working to manage the new “hybrid” work situations that have emerged from COVID. KTL’s work related to information management systems has grown as more and more companies need improved access to and control of information, regardless of where employees are working.
What would you say is a highlight of your job?
In my role, I have the opportunity to collaborate with and oversee our team of experts as they work with our clients to solve some really difficult problems. For example, we have seen an uptick in the number of surprise EPA inspections and enforcement actions over the past several months. In addition to preparing our clients to be “inspection-ready” as part of normal operations, our team has been able to respond quickly to provide resources that support our clients during and after these inspections.
We also have many clients who are working hard to implement best-in-class practices. These projects allow us to be creative in identifying approaches that help these organizations improve their transparency; develop more effective metrics and dashboards; and better align, focus, and manage those initiatives that are core to sustainable business operations.
What do you like to do in your free time?
My husband and I are adjusting to being empty nesters with two daughters now in college and out of the house. We recently bought a canoe and are looking forward to Wisconsin weather warming up enough to use it. I also really enjoy traveling and am continually planning for my next trip.
Read Lisa’s full bio.

Comments: No Comments
EPA’s Strategic Plan for the Future
On March 28, 2022, the Biden-Harris Administration submitted to Congress the President’s budget for fiscal year (FY) 2023 to support the Administration’s agenda to “build a better America, reduce the deficit, reduce costs for families, and grow the economy from the bottom up and middle out.”
Included in this budget is a historic investment of $11.881 billion to advance key priorities in the U.S. Environmental Protection Agency’s (EPA) FY 2022-2026 EPA Strategic Plan, including targeted objectives and outcomes in the areas of climate change; environmental justice; compliance enforcement; clean land, air, and water; and chemical safety.
Unprecedented Commitments
Every four years, EPA issues a Strategic Plan that communicates the Agency’s vision, priorities, and strategies for accomplishing its mission to protect human health and the environment. Since William Ruckelshaus served as the first Administrator of the EPA from 1970 to 1973, the Strategic Plan has been built on three foundational principles: follow the science, follow the law, be transparent.
The FY 2022-2026 Plan renews EPA’s commitment to these principles and adds a fourth to support Biden’s Justice40 initiative: advance justice and equity. Current EPA Administrator Michael Regan describes the EPA Strategic Plan as “bold and unprecedented in its commitment to advancing environmental justice and civil rights and tackling climate change.”
Roadmap to Protecting Human Health and the Environment
In the new Strategic Plan, EPA has established a roadmap to address climate change and environmental justice, in addition to its five programmatic areas of enforcement and compliance, air quality, water quality, land revitalization, and chemical safety. The Plan outlines the following seven long-term performance goals (LTPGs) and related objectives and quantifiable outcome to achieve by 2026.
Goal 1: Tackle the Climate Crisis
EPA is focused on further reducing greenhouse gas (GHG) emissions by promulgating rules to reduce pollution from the power sector, setting vehicle emission standards, and partnering with the public and private sectors communities (especially those underserved and disproportionally at risk) to increase energy efficiency in the residential, commercial, and industrial sectors.
In addition, there is $100 million in grants to support efforts to reduce GHG emissions and increase resiliency in the nation’s infrastructure and $35 million to implement the American Innovation in Manufacturing Act to continue phasing out GHGs.
Goal 2: Take Decisive Action to Advance Environmental Justice and Civil Rights
Environmental justice and civil rights will be embedded into EPA’s programs, policies, and activities to reduce disparities in environmental and public health conditions. This includes:
- Ensuring 80% of significant EPA actions with environmental justice implications clearly demonstrate how the action responds to environmental justice concerns.
- Identifying and implementing opportunities to integrate environmental justice and achieve civil rights compliance into planning, guidance, policy directives, monitoring, and review activities.
- Supporting Justice40 commitment to ensure at least 40% of the benefits of federal investments in climate and clean energy reach overburdened and underserved communities.
- Requiring all state recipients of EPA financial assistance to have foundational civil rights programs in place.
- Strengthening civil rights enforcement in communities with environmental justice concerns through civil rights and compliance reviews, audits, and community outreach.
- Proposing a new national environmental justice program office to coordinate and maximize the benefits of the Agency’s programs and activities.
Goal 3: Enforce Environmental Laws and Ensure Compliance
EPA intends to continue its enforcement path, holding environmental violators and responsible parties accountable. Significant investments are being made to enforce and assure compliance with the nation’s environmental laws, including $213 million for civil enforcement efforts, $148 million for compliance monitoring efforts, and $69 million for criminal enforcement efforts.
The Agency also has plans to improve inspections by sending 75% of EPA inspection reports to facilities within 70 days of inspection and conducting 55% of annual EPA inspections at facilities that affect communities with potential environmental justice concerns.
Goal 4: Ensure Clean and Healthy Air for All Communities
EPA has budgeted $1.1 billion to improve air quality and reduce localized pollution, reduce exposure to radiation, and improve indoor air for communities across the country. This plan includes reducing ozone season emissions of nitrogen oxides, improving measured air quality in counties not meeting the current National Ambient Air Quality Standards (NAAQS), reducing U.S. consumption of hydrofluorocarbons (HFCs), and preventing 2,250 lung cancer deaths annually through lower radon exposure.
Goal 5: Ensure Clean and Safe Water for All Communities
Approximately $4 billion is dedicated to upgrading the nation’s drinking water and wastewater infrastructure, with a focus on underserved communities. This includes reducing the number of community water systems still in noncompliance with health-based standards; leveraging EPA’s water infrastructure finance programs (CWSRF, DWSRF, WIFIA); providing Tribal, small, rural, or underserved communities with technical, managerial, or financial assistance to improve their drinking water or wastewater systems; and protecting and restoring waterbodies and watersheds. There are 20 new targeted water grant programs available to support EPA’s goal to ensure safe drinking water and reliable water infrastructure.
Goal 6: Safeguard and Revitalize Communities
Protecting communities from hazardous waste and environmental damage that can harm communities and that poses a risk to public health and safety continues to be a top priority. $1.15 billion is being allocated to EPA’s Superfund programs and $215 million for Brownfields programs to clean up and restore land to productive use, including:
- Bringing human exposures under control at an additional 60 Superfund sites.
- Completing 225 Superfund cleanup projects that address lead as a contaminant.
- Cleaning up an additional 650 brownfields properties.
- Making an additional 425 RCRA corrective action cleanups Sitewide Ready for Anticipated Use (SWRAU).
- Conducting an additional 35,000 cleanups at leaking underground storage tank (UST) facilities.
- Increasing the percentage of updated permits at RCRA facilities to 80%.
- Ensuring that 40% of annual emergency response and removal exercises that EPA conducts or participates in incorporate environmental justice.
Goal 7: Ensure Safety of Chemicals for People and the Environment
There is a large focus on strengthening EPA’s commitment and ability to successfully implement the Toxic Substances Control Act (TSCA). $124 million and 449 full-time equivalent (FTE) staff for TSCA efforts will support EPA-initiated chemical risk evaluations and regulations. This includes completing at least eight High Priority Substance TSCA risk evaluations annually and initiating TSCA risk management actions within 45 days of completing the evaluation.
In addition, EPA has set aside $126 million to increase its understanding of human health and ecological effects of per- and polyfluoroalkyl substances (PFAS) pollution, restrict its uses, and remediate PFAS that have been released.
Short-Term Focus
For the short-term, EPA has also identified three FY 2022-2023 Agency Priority Goals (APGs), which are intended to “jumpstart actions and showcase progress toward Administrator Regan’s priorities related to climate change, environmental justice, and civil rights” by September 30, 2023:
- Phase down the production and consumption of HFCs by 10% to be consistent with the schedule in the American Innovation and Manufacturing Act. This would decrease the U.S. consumption limit to less than 273.5 MMTCO2e in 2023.
- Deliver tools, guidance, metrics/indicators, and training for EPA and its Tribal, state, local, and community partners to advance environmental justice and external civil rights compliance.
- Clean up contaminated sites and invest in water infrastructure to enhance the livability and economic vitality of at least ten overburdened and underserved communities.
Set Your Goals
With EPA focused on more regulations, more enforcement, and improved climate change and environmental justice, environmental management needs to play an integral role in company strategy. Companies must take the time to be informed—particularly in the strategic areas described above—be prepared, and be proactive. Establish companywide priorities and goals and commit the appropriate resources to ensure programs and systems are in place to achieve regulatory compliance and align with EPA’s Strategic Plan.
If you would like help evaluating your current risk level and assessing your priorities, please contact KTL.

Comments: No Comments
Earth Day 2022: Invest in Our Planet
Every year on April 22, Earth Day celebrates the birth of the modern environmental movement in 1970. The very first Earth Day—spearheaded by Senator Gaylord Nelson—led to the creation of the U.S. Environmental Protection Agency (EPA) and the passage of new environmental laws, including the National Environmental Education Act, the Occupational Safety and Health Act, and the Clean Air Act.
In 1990, Earth Day went global, mobilizing 200 million people in 141 countries and bringing environmental issues to the forefront around the world. Today, Earth Day is recognized as the largest secular observance in the world, observed by more than a billion people—in classrooms, businesses, communities, governments across the world—as a day of action to protect our planet.
“A green future is a prosperous future.” ~ EARTHDAY.ORG
This year’s theme, “Invest in Our Planet,” focuses largely on sustainability and the growing climate crisis. According to EARTHDAY.ORG, “Smart companies are discovering that it is no longer a choice between going green and growing long-term profits — sustainability is the path to prosperity. […] it is imperative that companies of all sizes take action and embrace the benefits of a green economy.”
When businesses grow, they tend to follow a trajectory when it comes to environmental management and sustainability.

Leaders take the steps required to go beyond compliance and best practices to make commitments that embrace sustainability as a way of doing business—whether through efforts involving GHG reductions, beneficial reuse of products, recycling, sustainable materials management, land revitalization, sustainable food management, and more.
What You Can Do
For businesses, think about how you can transition into an environmental leader. KTL helps organizations every day to analyze processes and identify options for incorporating sustainable practices into daily operations—options that will not only work to protect the environment but also enhance reputation and protect the bottom line.
For individuals, Earthday.org shares 52 actions and tips to make a difference every day of the year.

Visit KTL at the 2022 Food Safety Summit
As one of the premier events in the food industry, the Food Safety Summit provides a comprehensive conference and expo for attendees to learn from subject matter experts, exchange ideas, and find solutions to current industry challenges. The Summit is back in person this year, and KTL is excited to be there live!
- When: May 9-12, 2022
- Where: Donald Stephens Convention Center, Rosemont, Illinois
- Who: Retailers, food processors, distributors, food manufacturers, growers, foodservice, testing laboratories, importing/exporting, law firms, and other food safety professionals
- Find KTL: Stop by our booth (#125) in the exhibit hall!
KTL Solutions Stage Presentation
Be sure to also update your agenda to attend KTL’s Solutions Stage presentation on Thursday, May 12 at 1:30 pm. KTL Principal Joe Tell and Partner and Senior Consultant Roberto Bellavia will be highlighting the following case study:
Food Safety Management System Case Study: Using Microsoft 365® to Improve Compliance
Food and beverage companies and their suppliers are subject to a wide range of complex regulatory and certification requirements, often with limited resources to maintain and demonstrate compliance. Finding effective information management tools is critical. Having a simple, centralized FSMS to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. The big secret: most companies already have the software they need in-house. KTL will present an industry case study demonstrating a cost-effective approach for building an FSMS using your existing Microsoft 365® platform with SharePoint®.

Comments: No Comments
FDA Issues Guidance for Voluntary Recalls
Foodborne illnesses impact millions of Americans every year—ranging from mild cases to hospitalizations to death. Reducing foodborne illness is a focus for the U.S. Food and Drug Administration (FDA), as reflected in several recent actions, including the proposed Food Traceability Rule (published on September 23, 2020) and the 2021 Foodborne Outbreak Response Improvement Plan (FORIP).
FDA recently took another step toward reducing the public’s exposure to the risks of foodborne illness on March 3, 2022, issuing its final guidance for voluntary recalls: Initiation of Voluntary Recalls Under 21 CFR Part 7, Subpart C.
About Voluntary Recalls
A voluntary recall involves actions taken by a company to correct or remove a violative product from the market, either on its own initiative or in response to a recommendation from the FDA. FDA Associate Commissioner of Regulatory Affairs Judith McMeekin, Pharm. D., emphasizes that “voluntary recalls continue to be the fastest, most effective way for a company to correct or remove violative and potentially harmful products from the market to help keep consumers safe.”
FDA’s new guidance outlines the steps companies should take before a recall is mandated, including developing policies and procedures, establishing training, maintaining records, and initiating communications.
BEFORE: Preparation Guidance
FDA’s guidance applies to any products that fall under the agency’s jurisdiction, including any food, drug, or device intended for human or animal use. The guidance breaks down the agency’s recommendations for activities companies (including manufacturers and distributors) should take in advance of a potential recall to be “recall ready.” These include the following:
- Establish a recall communications plan to address communications with employees, FDA, supply chain, direct accounts, and the public, as necessary. Identify key contacts and develop draft templates that can be easily customized and distributed when needed.
- Identify and train appropriate personnel (and alternates) on recall-related responsibilities. The recall team should have a thorough understanding of recall procedures and their respective roles in carrying out a recall plan. Regular training, including mock recalls, help ensure competency.
- Identify any reporting requirements for distributed products, if required by FDA. This might include a report to the Reportable Food Registry, an adverse event report for a dietary supplement, or a report to FDA upon product correction or removal.
- Establish and implement product coding to ensure traceability throughout production and distribution. This will enable more effective lot identification to accurately define and limit the recall scope.
- Maintain distribution records to allow for easier and faster identification of products to be recalled. Distribution records should include contact information for all direct accounts that received the product.
- Establish recall initiation procedures. Prepare, maintain, and document written procedures for initiating a recall to minimize delays and uncertainty when/if a voluntary recall becomes necessary. Procedures should be covered in training and should describe actions to carry out the following:
- Stop distribution, shipment, and/or sales of affected products.
- Outline a recall strategy that considers the scope/depth of the recall and associated risks.
- Notify direct accounts throughout the distribution chain and communicate instructions for appropriate disposition of product.
- Notify the public, when appropriate, if a product presents a health hazard.
DURING: Identifying and Initiating a Potential Recall
Identifying a potential problem is the first step in initiating a potential recall. It is vital that companies ensure timely identification and response to product problems that might lead to a recall. FDA suggests that the following indicators may suggest a potential concern:
- Internal reports of product specification deviation
- Out-of-specification testing results
- Consumer complaints
- Inspectional observations or laboratory results
- Reports of adverse events (e.g., illness, injury, death)
It is then the company’s responsibility to investigate the potential problem to determine 1.) whether a deviation has occurred, and 2.) whether the safety, effectiveness, purity, or potency of distributed products has been affected. A voluntary recall should not be delayed pending results of this investigation. Rather, companies should follow established procedures (see above) to decide whether to initiate a recall, determine the appropriate scope/depth of the recall, and resolve whether to discontinue production or distribution of impacted product(s).
To initiate a voluntary recall, companies should:
- Notify the FDA immediately if the company believes the product to be violative.
- Promptly issue recall communications to affected direct accounts and to the general public, if appropriate.
- Follow the procedures established in the company’s recall plan to implement the recall in accordance with 21 CFR 7.46.
- Provide instructions to the distribution chain regarding disposition of the product.
AFTER: Working with FDA
The FDA is committed to working with companies to help facilitate prompt response (i.e., removal or correction) to violative product that enters the marketplace. The agency has designated recall coordinators organized by product type located throughout the country who can work with company recall teams to develop strategies, review communications, monitor disposition of the product, coordinate with other regulatory bodies, etc.
The FDA may request a company initiate a recall under 21 CFR 7.45 after conducting discussions with the firm if the product presents a risk of illness, injury, or gross consumer deception; the firm has not initiated a voluntary recall; or agency action is required to protect public health.
Reducing Risks
The food industry and supply chains continue to change and evolve at an accelerated pace. That introduces new risks and challenges, including foodborne illness outbreaks. FDA’s most recent guidance is intended to help companies proactively address these risks—reducing response time in a foodborne illness outbreak and, subsequently, the number of people impacted.

Comments: No Comments
4 Quick Tips as EPA Inspections Continue
For the past several months, KTL has been talking about the significant uptick in U.S. Environmental Protection Agency (EPA) information surveys and multimedia inspections at regulated facilities—on our website, during webinars, at various conferences. Earlier this month, our team was called in to provide on-the-ground support to another manufacturer undergoing a comprehensive, unannounced multimedia inspection.
On the heels of this surprise inspection, one KTL Senior Consultant reiterates, “I can tell you firsthand based on this and other similar inspections over the past few months that inspectors are being very thorough and that the threat of financial penalty is substantial—particularly if your facility is not prepared.”
Take Action Now
KTL has been able to have some candid conversations with the EPA inspectors, and we know they plan to continue these unannounced inspections. EPA will be back in force this spring, and they have targets. It is in your best interest to make sure your facility is ready should an inspector show up on your doorstep.
Based on recent conversations and observations, KTL recommends taking at least the following four actions to prepare your facility:
- Verify your hazardous waste generator status in EPA’s Enforcement and Compliance History Online (ECHO) database and, if you are a small quantity generator (SQG), make sure that you have updated your EPA notification. All SQGs are required to update notification to EPA once every four years beginning September 2021. If you have not done this using the Form 8700-12, you are out of compliance and a likely target for an EPA inspection.
- Check the EPA EnviroFacts database to make sure you have identified emergency contacts for your facility and that the contacts are current. Inspectors are asking to see Emergency Response Plans and are particularly focused on correct contact information, clear conveyance of risk, and understanding of roles and responsibilities (i.e., training).
- Make sure you are appropriately managing your universal waste and hazardous waste. Have you completed a waste characterization for all hazardous waste streams and for solid waste streams that may appear to be hazardous? Are your containers closed, labeled with the nature of the hazard (see 40 CFR 262.16(b)(6)(i)(B), and dated? Are you appropriately managing and labeling any satellite accumulation areas (SAA) and container accumulation areas (CAA)?
- Establish a quick response internal inspection team that can evaluate all areas of risk in your facility to ensure you are in compliance at the time of inspection. Plan to check containers for proper labeling; ensure universal waste containers are closed and labeled properly; and verify that all hazardous waste, universal waste, and used oil management documentation is readily accessible. The inspector will ask to see your documentation.
KTL strongly encourages you to reach out if you have any questions or concerns about a potential EPA inspection. Our team has been through enough of these inspections with various clients recently to have a good handle on what to expect and what should be done to avoid financial penalty.

Comments: No Comments
Controlling Combustible Dust: Focus on Food
There is a wide range of agricultural and food products that create fire and explosion risks and hazards, including flour, grain, sugar, spices, cereal, flavoring additives, and many more. Dusts produced in any industry present challenges, but when manufacturing and processing food products, dusts can create the following major threats:
- Airborne dust can cause serious harm to human (employee) health by causing physical ailments ranging from dermatitis to occupational asthma to lung cancer.
- Dust can create the potential for cross contamination and encourage the spread of pathogens and allergens within the food processing plant.
- Dust may become combustible and serve as the source for explosions that harm workers, damage machinery, and destroy buildings/corporate reputation.
About Combustible Dust
The food industry is responsible for the largest percentage of combustible dust incidents in the U.S.—ranging anywhere from 24-43% depending on the timeframe and the source. One of the most notable combustible dust incidents in history involved a secondary explosion at the Imperial Sugar refinery in 2008, which killed 14 people and injured 40.
According to the Occupational Safety and Health Administration’s (OSHA) definition, combustible dust is “a solid material composed of distinct particles or pieces, regardless of size, shape, or chemical composition, which presents a fire or deflagration hazard when suspended in air or some other oxidizing medium over a range of concentrations.”
A dust explosion occurs when all five elements of the Dust Explosion Pentagon are present:
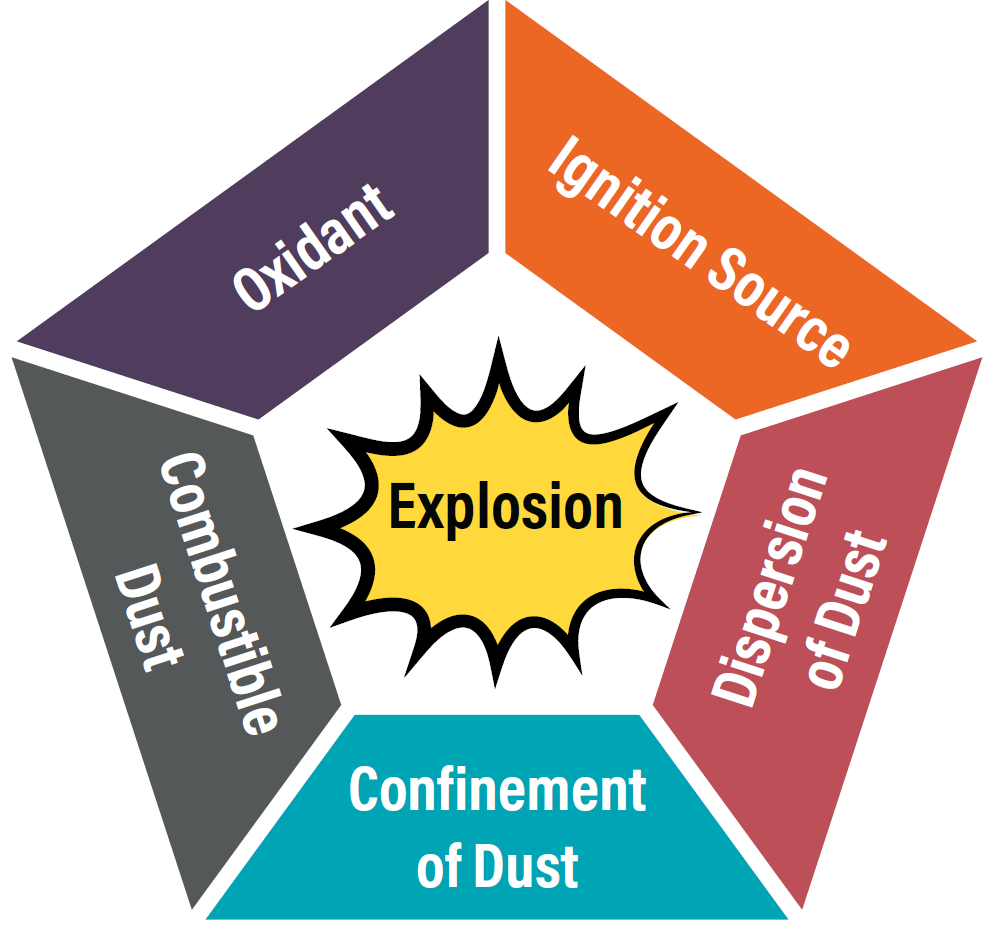
- Ignition source
- Oxygen
- Confined area
- Suspended cloud
- Explosive dust
Combustible Dust Standards
The devastating 2008 sugar refinery explosion prompted the development of OSHA’s Combustible Dust National Emphasis Program (NEP), and yet, some 14 years later, there is still no combustible dust regulation. That doesn’t mean, however, that there are no standards for managing and controlling combustible dust. In fact, there are several agencies involved in ensuring the food industry is appropriately managing the various hazards associated with combustible dust, including:
- National Fire Protection Association (NFPA)
- OSHA
- U.S. Food and Drug Administration (FDA)
NFPA Codes
NFPA sets standards regarding combustible dust. In fact, there are at least ten NFPA standards related to combustible dust (NFPA 61, 68, 69, 77, 484, 499, 652, 655, 664, 654). While these standards and codes are not law, per se, they are enforced by OSHA and referenced extensively. Most insurance agencies and local fire codes state that NFPA standards shall be followed as code.
NFPA’s most recent Standard on the Fundamentals of Combustible Dust (NFPA 652) covers the fundamental requirements for managing combustible dust fires and explosions and is an excellent starting point. NFPA 652 requires owners/operators to:
- Determine the dust’s combustibility and explosibility hazards based on laboratory testing or historical facility data.
- Conduct a Dust Hazard Analysis (DHA) to identify and evaluate potential dust fire and explosion hazards based on Kst (i.e., explosibility) values. Note: The DHA is a relatively new requirement—similar to OSHA’s Process Hazard Analysis as part of the Process Safety Management (PSM) program—used to assess risk and determine the required level of fire and explosion protection from combustible dust.
- Manage all the identified fire, flash fire, and explosion hazards identified in the DHA.
- Establish a written safety management system, including operating procedures and practices, training, incident investigation, and employee participation, to prevent and protect against the hazards.
NFPA 61 – Standard for the Prevention of Fires and Dust Explosions in Agricultural and Food Processing Facilities specifically covers facilities engaged in dry agricultural bulk materials or manufacturing and handling starch.
OSHA National Emphasis Program (NEP)
While OSHA has no formal standard for managing combustible dust, the agency published the Combustible Dust NEP in 2009, which outlines policies and procedures for inspecting workplaces that create or handle combustible dusts. The NEP heavily references the standards published by NFPA. It also references several other OSHA standards, including:
- 1910.22 Housekeeping
- 1910.307 Hazardous Locations
- 1910.1200 Hazard Communication
- 1910.269 Electric Power Generation, Transmission, and Distribution
- 1910.272 Grain Handling Facilities
- General Duty Clause, Section 5(a)(1) of the OSH Act
The General Duty Clause may be the most important of these, as it essentially gives OSHA the right to issue a citation any time employers fail to keep their employees safe from recognized hazards, including those hazards associated with combustible dust. Companies must control dust emissions to protect workers from exposure. If OSHA determines that even a very low Kst (i.e., low explosibility) dust is present in a facility with no explosion protection in place, the agency will issue citations and fines for lack of compliance.
Incidentally, OSHA published the Occupational Exposure to Flavoring Substances – Safety & Health Information Bulletin (SHIB) in 2010, cautioning that volatile organic compounds (VOCs) and respirable dust may be produced when handling many powdered flavoring formulations or spices. Inhalation of these substances may result in exposure directly to the small airways of the lungs.
FDA: Food Safety Modernization Act (FSMA)
In addition to meeting NFPA standards and OSHA guidelines, food companies must meet the requirements of FDA’s Food Safety Modernization Act (FSMA). FSMA requires food processing facilities to implement measures to ensure contamination hazards will be minimized or prevented. This includes developing a Hazard Analysis and Critical Control Points (HACCP) Plan with preventive controls for processes, food allergens, foreign objects, sanitation, and supply chain, as well as complying with Good Manufacturing Practices (GMPs).
Best Practices
Combustible dust itself is not avoidable, but the related consequences are. When it comes down to it, poor housekeeping is the biggest root cause of combustible dust issues. Many risks and hazards can be avoided by effectively cleaning the facility and equipment to remove contaminants before they become widely dispersed. Diligent housekeeping, combined with installing a dust collection system that is properly designed for the operation, can significantly reduce airborne dust and help mitigate a primary or more deadly secondary explosion.
If you work in the food industry, combustible dust is a concern. Make sure you understand the applicability of all the standards and codes, particularly the requirements under the various NFPA standards. The following may be required to keep your facility in compliance and your employees safe:
- Laboratory screening to determine if your powders are combustible or explosible.
- DHA to identify and evaluate potential dust fire, flash fire, and explosion hazards.
- Full analytical testing to determine explosion strength (Kst), minimum ignition energy, maximum safe storage temperature, and more.
- A written management system that documents operating procedures and practices for the ongoing management of dust fire, flash fire, and explosion hazards.
- Initial and ongoing training for operating, engineering, and management staff to build competency and understanding.

Comments: No Comments
KTL Consultant April Greene Achieves BCSP Certification

Consultant April Greene has become KTL’s newest Certified Safety Professional (CSP). April has completed all experience and eligibility requirements—including recently passing the rigorous CSP exam—for a Board of Certified Safety Professionals® (BCSP®) certification. The CSP is one of the industry’s most recognized environmental, health, and safety (EHS) certifications.
April is an experienced EHS professional with a 10-year history of working in the EHS services industry and laboratory settings. She has worked with many of KTL’s clients to create and manage EHS programs, plans, policies, and procedures to ensure regulatory compliance and workplace safety. In addition to her BCSP certification, April is a certified ISO 45001 (safety) and 14001 (environment) Lead Auditor and Wisconsin Department of Natural Resources (WDNR) Green Tier Auditor.
Safety issues have become more complex, and today’s safety professional must continually be better qualified. “Safety, health, and environmental practice relies on the knowledge and skills of its practitioners,” explains BCSP’s CEO, Christy Uden, CAE, IOM.
BCSP credential holders are among the most highly trained, educated, and experienced individuals in the safety field. Having achieved a BCSP certification demonstrates April’s proven knowledge of EHS fundamentals, her commitment to worker safety, and the professionalism of her safety practice.
Next up for April: First a new tattoo to commemorate her CSP (think GHS pictograms), and then she’s off to the BCSP Global Learning Summit in May.

Comments: No Comments
Staff Spotlight on Evan Fitzgerald
Get to know our KTL team! This month, we are catching up with KTL Principal Evan Fitzgerald. Evan leads KTL’s efforts to help clients develop, automate, and improve management and compliance information systems and optimize them to reduce costs and improve performance. He has developed numerous cloud-based applications that KTL uses to deliver compliance efficiency tools to our clients. He is based out of KTL’s Madison, WI headquarters.
Tell us a little bit about your background—what are your areas of expertise?
I earned a degree in mechanical engineering in 1996 and went to work for RMT, an environmental consulting firm, with the plan of being on their process engineering team. I ended up doing mostly construction management, which was not the direction where I saw growth in environmental compliance. When an opportunity with Kestrel Management (now KTL) opened in 1998, I jumped at the chance to make the switch to a firm that viewed environmental compliance from a management and process perspective. That made me employee #1 with the five original owners.
Since then, I’ve done a little bit of everything at KTL—but always with an eye towards technology. That included running our Aerie Technologies business when we created a joint venture to take the dynaQ™ assessment software into the marketplace. It was during that time I first met KTL Principal Joe Tell (formerly of Tellevate LLC) at a trade show focusing on software in environmental management. It was years in the making, but on November 1, 2019, Kestrel Management and Tellevate LLC merged to form KTL.
I now primarily focus on managing the SharePoint/IT side of KTL and have also taken on more business management responsibility as we have transitioned our management team. I have never been as excited and hopeful for the direction of KTL given the staff and clients we have put together.
What types of clients do you work with? What are the biggest issues you see them facing right now?
I work with all types of KTL clients. The biggest issue facing them (from my view of things) is the same thing that’s been facing them for decades—a need to improve processes via the use of technology with little direction and support provided by their internal resources.
People often ask me who our biggest competitor is in the IT sector, and I always say our biggest competitors remain Microsoft Excel and Word. Our clients do not have the time to put to use the tools often already at their disposal, and switching to one of the large platforms is expensive and time-consuming. KTL fills that gap by developing the right level of tool built to our clients’ current operations.
What would you say is a highlight of your job?
The diversity of work and clients that we help at KTL. It’s pretty rare when any two days are the same…and I don’t think after 23 years here that I’ve ever had two weeks the same.
What do you like to do in your free time?
My wife and I have three children that have kept us pretty busy for the past 21 years between theater, sports, and school functions. Our youngest is still in the house and has taken up competitive volleyball, giving us our first taste of the weekend sports tournament grind.
I have been an avid strategy card game and board game player for about 25 years. My big summer trip was attending a massive gaming convention in Indianapolis called GenCon. That’s taken a dip due to COVID. Hopefully, that can get back on track soon. I also participate in an archery and trap shooting league throughout the year.
Read Evan’s full bio.
