
Comments: No Comments
EPA Announces Chemical Safety Milestones
EPA Announces Chemical Safety Milestones
To celebrate the one-year anniversary of the Frank R. Lautenberg Chemical Safety for the 21st Century Act, EPA Administrator Scott Pruitt announced on June 22, 2017, that the Agency has met its first-year statutory responsibilities under the law. This includes the following actions:
- Issuing a rule to establish EPA’s process and criteria for identifying high-priority chemicals for risk evaluation and low-priority chemicals not requiring risk evaluation. http://www.epa.gov/assessing-and-managing-chemicals-under-tsca/prioritizing-existing-chemicals-risk-evaluation
- Issuing a rule to establish EPA’s process for evaluating high-priority chemicals to determine whether they present an unreasonable risk to health or the environment. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluations-chemicals-under-tsca
- Issuing a rule to require industry reporting of chemicals manufactured or processed in the U.S. over the past 10 years. https://www.epa.gov/tsca-inventory/tsca-inventory-notification-active-inactive-rule
- Releasing scope documents for the initial ten chemicals for risk evaluation under the amended law. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluations-chemicals-under-tsca#ten
- Releasing guidance for external parties interested in submitting draft risk evaluations to the EPA for consideration. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/guidance-assist-interested-persons-developing-and
Read the EPA press release: https://www.epa.gov/newsreleases/epa-marks-chemical-safety-milestone-1st-anniversary-lautenberg-chemical-safety-act

Environment / Food Safety / Quality / Safety / Technology Enabled Business Solutions
Comments: No Comments
Technology Tip: Software and Audits Top 10
All types of business and operational processes demand a variety of audits and inspections to evaluate compliance with standards—ranging from government regulations to industry codes, to system standards (i.e., ISO), to internal corporate requirements.
Audits provide an essential tool for improving and verifying compliance performance. Audits may be used to capture regulatory compliance status, management system conformance, adequacy of internal controls, potential risks, and best practices.
By combining effective auditing program design, standardized procedures, trained/knowledgeable auditors, and computerized systems and tools, companies are better able to capture and analyze audit data, and then use that information to improve business performance. Having auditing software of some sort can greatly streamline productivity and enhance quality, especially in industries with many compliance obligations.
The following tips can help ensure that companies are getting the most out of their auditing process:
- Have a computerized system. Any system is better than nothing; functional is more important than perfect. The key is to commit to a choice and move forward with it. Companies are beginning to recognize the pitfalls of “smart people” audits (i.e., an audit conducted by an expert + notebook with no protocols or systems). While expertise is valuable, this approach makes it difficult to compare facilities and results, is not replicable, and provides no assurance that everything has been reviewed. A defined system and protocol helps to avoid these pitfalls.
- Invest time before the audit. The most important time in the audit process is before the audit begins. Do not wait until the day before to prepare. There is value in knowing the scope of the audit, understanding expectations, and developing question sets/protocol. This is also the time to ensure that the system collects the data desired to produce the final report.
- Capture data. Data is tangible. You can count, sort, compare and organize data so it can be used on the back end. Data allows the company to produce reports, analytics, and standard metrics/key performance indicators.
- Don’t forget about information. Information is important, too. The information provides descriptions, directions, photos, etc. to support the data and paint a complete picture.
- Be timely. Reports must be timely to correct findings and demonstrate a sense of urgency. Reports serve as a permanent record and begin the process of remediation. The sooner they are produced, the sooner corrective actions begin.
- Note immediate fixes. During the audit, there may be small things uncovered that can be fixed immediately. These items need to be recorded even if they are fixed during the audit. Unrecorded items “never happened”. Correspondingly, it is important to build a culture where individuals are not punished for findings, as this can result in underreporting.
- Understand the audience. Who will be reading the final report? What do they need to know? What is their level of understanding? Not all data presentation is useful. In fact, poorly presented data can be confusing and cause inaction. It is important to identify key data, reports desired, and the ways in which outputs can be automated to generate meaningful information.
- Compare to previous audits. The only way to get an accurate comparison is if audits have a common scope and a common checklist/protocol. Using a computerized system can ensure that these factors remain consistent. Comparisons reinforce and support a company’s efforts to maintain and improve compliance over time.
- Manage regulatory updates. It is important to maintain a connection to past audits and the associated compliance requirements at the time of the audit. Regulations might change and that needs to be tracked. Checklists, however, may remain the same. Companies should have a process for tracking regulatory updates and making sure that the system is updated appropriately.
- Maintain data frequency. For data, the frequency is key. Consider what smaller scope, higher frequency audits look like. These can allow the company to gather more data, involve more people, and improve the overall quality and reliability of reports.
A well-designed and well-executed auditing program—with analysis of audit data—provides an essential tool for improving and verifying business performance. Audits capture regulatory compliance status, management system conformance, adequacy of internal controls, potential risks, and best practices. And using a technology tool or system to manage the audit makes that information even more useful.

Comments: No Comments
EPA Proposes to Delay RMP Rule Effective Date to 2019
EPA Proposes to Delay RMP Rule Effective Date to 2019
On Friday, March 31, 2017, U.S. Environmental Protection Agency (EPA) Administrator Scott Pruitt announced a proposed rule to further delay the effective date of the Obama Administration’s Risk Management Program (RMP) final rule until February 19, 2019. This will give the agency time to reconsider the final RMP rule published on January 13, 2017.
Industry organizations have raised serious concerns about the final rule. The proposal to further delay the effective date of the amendments will allow the Agency time to evaluate these objections and consider other issues that may benefit from the additional public input.

Comments: No Comments
EPA Puts Risk Management Program Rule on Hold
EPA Puts Risk Management Program Rule on Hold
This January, the much anticipated final RMP amendments were published in the Federal Register. According to the EPA, these amendments are intended to:
- Prevent catastrophic accidents by improving accident prevention program requirements
- Enhance emergency preparedness to ensure coordination between facilities and local communities
- Improve information access to help the public understand the risks at RMP facilities
- Improve third-party audits at RMP facilities
As Kestrel indicated in a recent article when the final RMP amendments were published, RMP faces an uncertain future under the Trump Administration. It is not clear at this point whether the final RMP rule will actually be implemented as published—or at all.
We are seeing the first wave of that uncertainty demonstrated. EPA received a petition dated February 28, 2017, from the RMP Coalition requesting a reconsideration and request for a stay for the RMP rule amendments. After a proceeding for reconsideration on March 13, 2017, EPA’s Administrator signed a final rule that provides a three-month (90-day) administrative stay of the effective date of the RMP rule amendments, delaying the effective date of the final rule to June 19, 2017. This stay is intended to allow the EPA to revisit these important issues and consider alternative approaches.

Comments: No Comments
Risk Management Plan (RMP) Final Amendments
Chemicals are an important part of many aspects of our lives; however, improper handling and management of chemicals can result in catastrophic releases that have severe and lasting impacts—loss of life, injury, property damage, community disruption. USEPA’s Risk Management Plan (RMP) data shows that in the last 10 years, there have been more than 1,517 reportable incidents of accidental chemical releases. Those incidents were responsible for 58 deaths, 17,099 injuries, the evacuation or shelter-in-place of almost 500,000 people, and over $2 billion in property damage.
Charting the Changes to RMP
The USEPA’s RMP Rule (Section 112(r) of the Clean Air Act Amendments) is aimed at reducing the frequency and severity of accidental chemical releases. Changes to the RMP Rule have been in progress since former President Obama issued Executive Order (EO) 1365, Improving Chemical Safety and Security, in August 2013. The focus of the EO is to reduce risks associated with hazardous chemicals to owners and operators, workers, and communities by enhancing the safety and security of chemical facilities. Modernizing policies and regulations—including the RMP Rule—falls under this umbrella.
A July 2014 Request for Information (RFI) sought initial comment on potential revisions to RMP under the EO. This was followed by a Small Business Advocacy Review (SBAR) Panel discussion in November 2015. On March 14, 2016, the USEPA published Proposed Rule: Accidental Release Prevention Requirements: Risk Management Programs Under the Clean Air Act, Section 112(r)(7), outlining proposed amendments to the RMP Rule.
Since the initial action to revise the RMP Rule commenced two and a half years ago, the USEPA has received over 60,000 public comments and has had extensive engagement with nearly 1,800 people.
Final Amendments
The much anticipated final RMP amendments were published in the Federal Register on January 13, 2017. According to the USEPA, these amendments are intended to:
- Prevent catastrophic accidents by improving accident prevention program requirements
- Enhance emergency preparedness to ensure coordination between facilities and local communities
- Improve information access to help the public understand the risks at RMP facilities
- Improve third-party audits at RMP facilities
The final changes in the RMP Rule are outlined in the table below.
Compliance
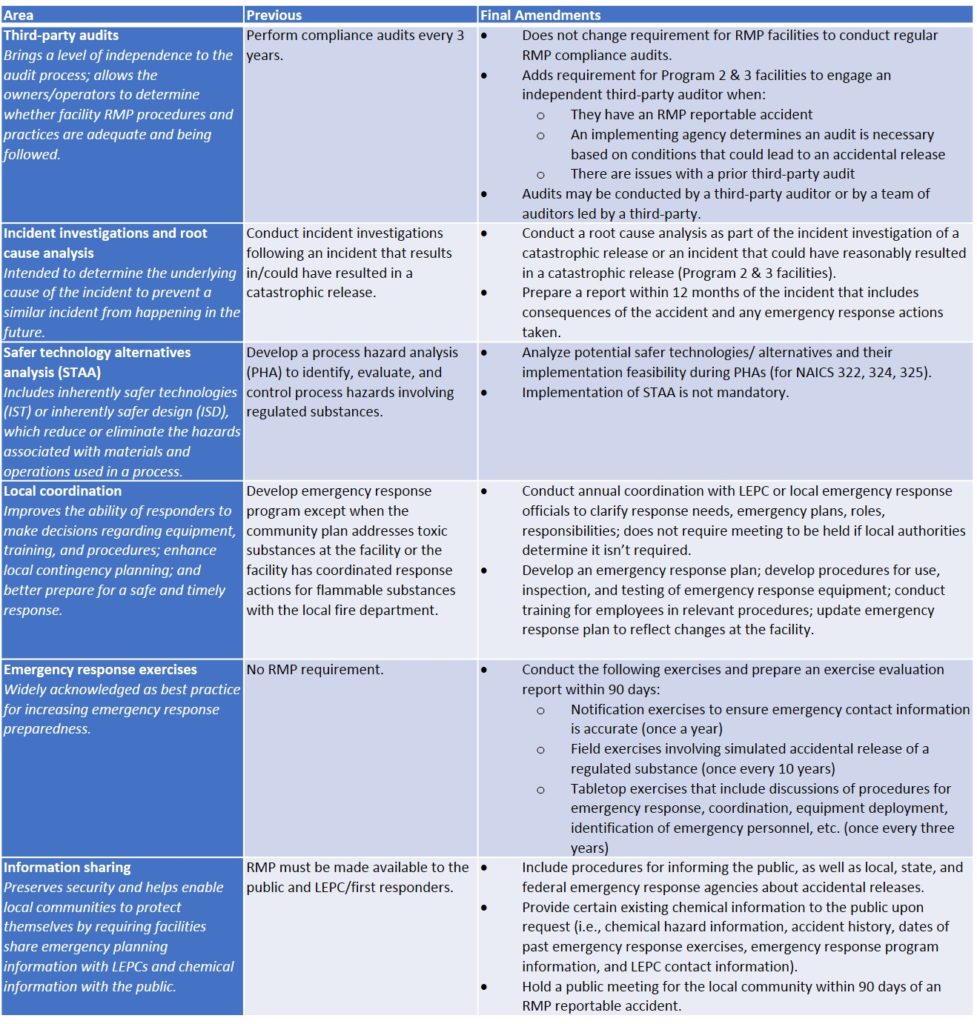 The effective date for the final RMP amendments is March 14, 2017. Compliance dates are set according to this date, as follows:
The effective date for the final RMP amendments is March 14, 2017. Compliance dates are set according to this date, as follows: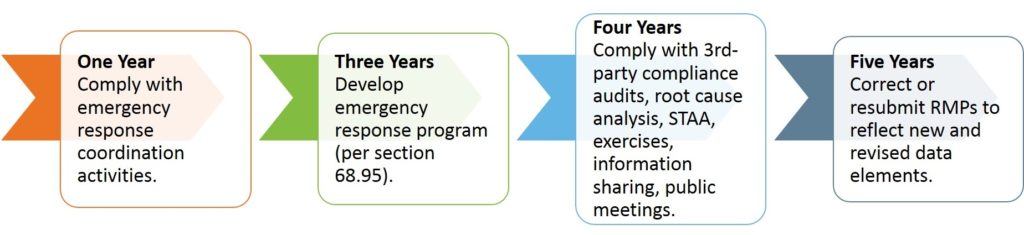
The Future of RMP
The final RMP amendments have the potential to significantly affect the 12,500 facilities in the U.S. that are subject to the RMP program. However, like many environmental rules, RMP faces an uncertain future under the Trump Administration. It is not clear at this point whether the final RMP Rule will actually be implemented as published—or at all.
Among the possible outcomes, environmental law firm Beveridge & Diamond PC cites the following possibilities:
- Congress may rescind the Rule using the Congressional Review Act.
- The USEPA might stay the Rule and then unilaterally seek to repeal it through amendment.
- The Rule might be challenged through a petition for reconsideration to the USEPA or a petition for review by the federal courts.
Kestrel will continue to track the RMP Rule and potential upcoming actions or compliance dates that may affect impacted facilities.

Case Study: Efficient Compliance Management
Regulatory enforcement, customer and supply chain audits, and internal risk management initiatives are all driving requirements for managing regulatory obligations. Many companies—especially those that are not large enough for a dedicated team of full-time EHS&S staff—struggle with how to effectively resource their regulatory compliance needs.
The following case study talks about how The C.I. Thornburg Co., Inc. (C.I. Thornburg) is using a technology tool to efficiently meet National Association of Chemical Distributors (NACD) and a number of other regulatory requirements.
The Challenge of Compliance
C.I. Thornburg joined NACD in January 2015. As a condition of membership, the company started the process of developing and implementing Responsible Distribution in April 2015. Responsible Distribution showcases member companies’ commitment to continuous improvement in every business process of chemical distribution—and it requires rigorous management activities to develop and maintain.
With an EHS&S department of one, managing all of those activities was a challenge for C.I. Thornburg. The company was looking for a way to streamline the process and more effectively manage Responsible Distribution requirements and regulatory compliance obligations.
Code & Compliance Elite™
C.I. Thornburg brought on Kestrel to initially help the company achieve Responsible Distribution verification. Kestrel worked with C.I. Thornburg to customize and implement Code & Compliance Elite (CCE™), an easy-to-use technology tool designed to effectively manage management system and verification requirements. Kestrel tailored the CCE™ application specifically for C.I. Thornburg to provide:
- Document management – storage, access, and version control
- Mobile device access
- Regulatory compliance management and compliance obligation calendaring
- Internal audit capabilities
- Corrective and preventive action (CAPA/CPAR) tracking and management
- Task and action management
CCE™ played a large role toward the end of C.I. Thornburg’s Responsible Distribution implementation, particularly with document control and organization, and in the verification audit. During verification, documents could be quickly referenced because of how they are organized in CCE™, making the process very efficient. According to C.I. Thornburg Director of Regulatory Compliance and EHS&S Richard Parks, “The verifier was blown away by how well we were organized and how the tool linked many documents from different regulatory policies.” The company achieved verification in May 2016.
Broadening to Other Regulatory Requirements
CCE™ is still being used to manage Responsible Distribution requirements, but C.I. Thornburg is now working with Kestrel to expand it to all regulatory branches that govern the business. Regulatory requirements function similarly—for example, Responsible Distribution has 13 codes, Department of Homeland Security (DHS) has 18 performance standards (RBPS), and OSHA PSM has 14 elements. All require internal audits and corrective action tracking—things that can be easily and effectively managed through CCE™ to create a one-stop shop for regulatory compliance. Kestrel is currently developing the DHS and PSM modules in CCE™ for C.I. Thornburg.
Valuable Management Tool
CCE™ is providing C.I. Thornburg with a valuable management tool that automates the regulatory landscape. According to Parks, as a small organization that depends on using efficient tools to manage compliance rather than adding more manpower, CCE™ has provided huge cost savings and tremendous value for the organization, including the following:
- CCE™ has become the ultimate tool inefficiency. Tasks that used to take hours to complete are now easily done in just minutes.
- The internal audit function of CCE™ makes audits seamless and tracking and follow-up easy.
- The CAPA tool ensures that the company is managing corrective actions and completing follow-up activities and tasks.
- The functionality of CCE™ allows for managing multiple regulatory dashboards, providing a one-stop shop for managing regulatory compliance obligations.
- CCE™ creates an organized document structure that enables easy access to information and quick response to auditors.
- During Senior Management Review, senior managers see the benefit of being able to reference the history of corrective actions and audits through CCE™.
“A lot of NACD member companies are small organizations that have limited resources to effectively manage all EHS&S needs,” said Parks. “CCE™ really creates the department and is a huge value to small businesses. With the CCE™ technology and a company’s clearly defined goal, Kestrel can provide an efficient solution to most any need.”

Comments: No Comments
Frank R. Lautenberg Chemical Safety Act
Last year, we came to you with breaking news about Toxic Substances Control Act (TSCA) reform taking hold, as the U.S. House of Representatives passed the TSCA Modernization Act of 2015 (H.R. 2576) on June 23, 2015.
Almost one year later—and approximately 40 years since the Act’s inception—President Obama signed the Frank R. Lautenberg Chemical Safety Act (FRL-21) into law on June 22, 2016, amending the nation’s primary chemical management law. A historic bipartisan achievement, this Act gives the USEPA immediate authority to begin evaluating the risk of any chemical it designates as “high priority”.
Background
TSCA was developed to ensure that products are safe for intended use by providing the USEPA authority to review and regulate chemicals in commerce. Despite its intention, TSCA has proven to be rather ineffective in providing adequate protection and in facilitating U.S. chemical manufacturing and use. More than 80,000 chemicals available in the U.S. have never been fully tested for their toxic effects on health and the environment. In fact, under TSCA, the USEPA has only banned five chemicals since 1976.
According to a blog by USEPA Administrator Gina McCarthy, “While the intent of the original TSCA law was spot-on, it fell far short of giving EPA the authority we needed to get the job done.”
And that is where FRL-21 takes over, strengthening the foundation built by TSCA to ensure that chemical safety remains paramount.
Key Changes
FRL-21 remains consistent with the 2009 Principles for TSCA Reform. The USEPA outlines the following key regulatory changes in its Q&A briefing on the Act.
Evaluates the safety of existing chemicals in commerce, starting with those most likely to cause risks. This is the first time that all chemicals in commerce will undergo risk-based review by the USEPA. The Agency is charged with creating a risk-based process to determine which chemicals should be prioritized for assessment. High-priority chemicals may present an unreasonable risk to health or the environment due to potential hazard and route of exposure. A high-priority designation, in turn, triggers a risk evaluation to determine the chemical’s safety. This prioritization ensures that those chemicals that present the greatest risk will be reviewed first.
Evaluates new and existing chemicals against a new risk-based safety standard. Under the law, the USEPA will evaluate chemicals based purely on the health and environmental risks they pose. The evaluation must also include considerations for vulnerable populations (e.g., children, elderly, immune-compromised). FRL-21 further repeals the requirement that the Agency apply the least burdensome means of adequately protecting against unreasonable risk from chemicals. Costs and benefits will not be factored into the evaluation.
Empowers USEPA to require the development of chemical information necessary to support these evaluations. In short, the Agency has expanded authority to demand additional health and safety or testing information from manufacturers and/or to conduct risk evaluations on a chemical. USEPA may also expedite the process through new order and consent agreement authorities.
Enforces clear and enforceable deadlines that ensure timely review of prioritized chemicals and timely action on identified risks. Strict deadlines are designed to keep the USEPA’s work on track and to ensure compliance by manufacturers. For example, the Agency must have 10 ongoing risk evaluations within the first 180 days and 20 ongoing risk evaluations within 3.5 years. When unreasonable risks are identified, USEPA must then take final risk management action within two years. Action, which may include labeling, bans, and phase-outs, must begin no later than five years after the final regulation.
Increases public transparency of chemical information by limiting unwarranted claims of confidentiality. The USEPA must review and make determinations on all new confidentiality claims for chemical identity, as well as review past confidentiality claims to determine if they are still warranted. This will allow companies to preserve their intellectual property and competitive advantage, while still providing transparency to the public.
Provides a source of funding for the USEPA to carry out these changes. The USEPA can collect up to $25 million annually in user fees from chemical manufacturers and processors when they:
- Submit test data for USEPA review
- Submit a pre-manufacture notice for a new chemical
- Manufacture or process a chemical that is the subject of a risk evaluation
- Request that the USEPA conduct a chemical risk evaluation
Impacts
For companies, the most immediate impacts of FRL-21 will be on the new chemicals review process, as the USEPA has to approve any new chemical or significant new use of an existing chemical before manufacturing can commence and chemicals can enter the marketplace. This process will help provide regulatory certainty throughout the supply chain—from raw material produces to retailers. And, in the end, the risk evaluations will help ensure that manufacturers are able to bring new chemicals to the market in a safe and efficient way.
As for the general public, FRL-21 creates a new standard of safety to protect the public and the environment from unreasonable risks associated with chemical exposure. For the first time in 40 years, it provides assurance and greater confidence that chemicals are being used safely.

Environment / Quality / Safety
Comments: No Comments
Management System Internal Audit: What to Expect
Many companies face requirements to conduct management system internal audits. And many probably consider it to be one of those “necessary evils” of doing business. In reality, an internal audit can be a great opportunity to uncover issues and resolve them before an external audit begins. An internal audit can sometimes even enable more improvements than an external audit because it allows the company to review processes more often and more thoroughly. So what, exactly, goes into an internal audit?
What Is an Audit?
First, conducting a management system internal audit encompasses all of the efforts to gather, accumulate, arrange, and evaluate data so that there is sufficient information to arrive at an audit opinion. According to the ANSI/ASQC Standard Q1-1986 Generic Guidelines for Auditing Management Systems, an audit is:
a systematic examination of the acts and decisions by people with respect to Q/EHS issues, in order to independently verify or evaluate and report conformance to the operational requirements of the program or the specification or contract requirement of the product or service.
Internal audits should be carried out to look for areas for improvement and best practices. In an internal audit, the auditor is evaluating, verifying, and reporting conformance or non-conformance in terms of related documentation. The auditor assesses systems, processes, and products against the related documentation:
- Systems are compared against company directives and requirements.
- Processes are compared against procedures, process charts, and work instructions.
The auditor examines where and how “operational requirements of the management system” are described. This is done by reviewing each policy, procedure, work instruction, checklist, and form looking for each “actionable item” listed within.
The Interview
The auditor will go out into the workforce and ask the prepared questions to various employees. Based on the responses given, the auditor may need to ask follow-up questions to get a clear understanding of how an operation works. Questions asked by auditors are generally open-ended to give the auditee the opportunity to elaborate. The auditor’s goal is to give the employee the opportunity to think prior to answering and to follow the audit trail wherever it leads—within or outside of the department.
Tangible Evidence
In order for an internal audit to support improvement steps, the auditor will seek tangible evidence. For example, work instructions require that inspections are completed every day, but the checklist shows that no checks have been performed for the last week. Tangible evidence may include taking a photo copy of the checklist to document this issue.
Evaluating Internal Controls
During the audit, the auditor is looking for internal controls that regulate an operation. There are seven steps in evaluating internal controls:
- Observe the Operation: The auditor needs to understand what processes and systems to review, where they are located, and who is responsible for them.
- Identify Constraints: The auditor will identify constraints to the extent possible, such as:
- Scattered information
- Internal opposition
- Process not capable
- Process not in control
- Unavailable information
- Evaluate Risk: The auditor will assess the importance and risk of internal controls not detecting and preventing non-conformances. The auditor will ask personnel being audited and management if there is anything more that could be done to identify and control risk.
- Evaluate the Internal Control Structure: Usually extensive internal controls exist, operate properly, and maintain/improve the process; however, this may not be an accurate assumption. Controls may not exist, may be weak, or may control and measure unimportant variables. It is very important for the auditor to resist assuming that the way an existing system has been set up is the correct way to do something. Auditors should challenge how and why something is being done to encourage system improvements.
- Test the Effectiveness of the Internal Control Structure: Gathering evidence is the process of collecting data and information critical to support a decision or judgment rendered by the auditor.
- Evaluate Evidence: Once evidence has been gathered from interviews, observations, or records, the auditor must distill and summarize the data into useful information for the company. The evidence is then reviewed to determine whether systems and controls are working effectively.
- Issue an Opinion: When all is said and done, the auditor must issue an opinion of conformance or non-conformance. In a deficiency finding (non-conformance), the audit report will clearly state that there is a variance between what is and what should be. All evidence findings should be listed to support this conclusion.
Clarify Issues and Non-Conformances
Upon completion of an audit, there may be times when clarification of an issue or concern will be warranted. This is when the auditor may go back to the department head and review the current understanding of the audit results. The department head should have ample time to discuss and clarify any issues of concern.
Any outstanding issues that warrant a non-conformance report should be discussed to ensure that the company understands: 1.) why the issue is considered a non-conformance, and 2.) what may need to be done to rectify the situation. It is important to also discuss all positive findings from the audit to leverage best practices.
By using an internal audit to actually improve operations—and not just as another requirement to fulfill—companies can realize significant value through:
- Meeting regulatory/certification requirements prior to the external audit
- Improving operational controls and processes
- Enhancing overall management system effectiveness

Comments: 1 Comment
Hazardous Materials Management Plan (HMMP)
An ever-growing area of concern across the industry is the appropriate management of hazardous materials and waste. The number of government regulations concerning hazardous materials handling alone is significant—from the U.S. Environmental Protection Agency (USEPA) and Occupational Safety and Health Administration (OSHA), to the Department of Transportation (DOT), Department of Homeland Security (DHS), Drug Enforcement Agency (DEA), and Nuclear Regulatory Commission (NRC). Under these laws, the disposal of hazardous material in the sewer system, stormwater system, on the ground, or in regular trash is regulated by a number of prohibitions.
In addition to these regulations, there are international codes for hazardous materials —developed by the International Fire Code (IFC)/International Building Code (IBC) and operationalized by local officials—that apply across the industry. Kestrel has found that more and more facilities, particularly those in retail warehousing, are being subjected to the requirements of these codes.
IFC/IBC Requirements
Local fire marshals have the responsibility to inspect facilities and ensure that hazardous materials are properly managed and stored. Although local requirements may vary, most local laws have closely adopted the IFC/IBC requirements for hazardous materials management.
The IFC/IBC requirements reference hazardous material as it relates to hazard classes—a significant difference compared to some of the other hazardous materials regulations across the U.S. Applicability and specific requirements are based on:
- Hazard class of the chemical
- Volume of the chemical
- How the chemical is used
- How the chemical is stored
- Building occupancy
Depending on these things, the local fire marshal may require the facility to develop a Hazardous Materials Management Plan (HMMP), conduct a Hazardous Materials Inventory, and/or submit a Hazardous Materials Permit.
HMMP Requirements
The purpose of a Hazardous Materials Management Plan (HMMP) is to describe the company’s procedures for storing, using, managing, and disposing of hazardous materials in a safe manner.
The information requested for an HMMP is different than that required for Tier II reporting under Section 312 of the Emergency Planning and Community Right-to-Know Act (EPCRA). The purpose of the Tier II form is to provide state and local officials and the public with information on the general hazard types and locations of hazardous chemicals at the facility.
Notable differences between Tier II and HMMP include the following:
- Classifications and regulations are different.
- Volumes that trigger an HMMP are much lower.
- Use and storage of the chemical, as well as the occupancy of the building, influence the requirements for an HMMP.
Beyond the HMMP, a company may need to conduct a Hazardous Materials Inventory, which requires defining hazard classes for chemicals at the facility and may need to apply for a Hazardous Material Permit under the authority of the local fire marshal.
While there may be variations by the local authority, in general, the Plan and Inventory include the following:
| Hazardous Materials Management Plan (HMMP) | Hazardous Materials Inventory |
|
|
Hazard Classes and MAQs
Hazard classes, as required in the Hazardous Materials Inventory, are listed in IFC Appendix E. They include physical hazards and health hazards, and typically have a number of associated subcategories. A hazard class is not listed on a Safety Data Sheet (SDS). The classes include:
- Explosives
- Compressed Gases
- Flammable and combustible liquids
- Flammable solids
- Combustible dust and powders
- Oxidizers
- Organic Peroxides
- Pyrophoric materials
- Unstable materials
- Water reactive materials
- Cryogenic materials
- Toxic materials
- Corrosives
The MAQ for a hazardous material will determine occupancy requirements and required controls (sprinkler systems, temperature controlled storage etc.). The MAQs are located in:
- IBC Table 307.1(1) and IFC Table 5003.1.1(1) for physical hazard hazardous materials
- IBC Table 307.1(2) and IFC Table 5003.1.1(2) for health hazard hazardous materials
- IFC Tables 5003.1.1(3) and (4) for outdoor control area MAQ
Note that the IBC and IFC are both involved to determine the hazard classification and the associated storage limits and requirements.
Moving Forward
Companies with hazardous materials must manage their hazardous materials safely. To ensure that they are meeting the IFC/IBC requirements, it is beneficial for companies to:
- Check with the local fire marshal to determine what needs to be done to meet local requirements, including engineering design requirements.
- Prepare and maintain a facility map identifying storage areas, sprinkler systems, emergency plans, etc. and document it in an HMMP.
- Contact vendors/installers of sprinkler systems and other equipment to ensure that the systems are designed for their intended use. The installer is a good resource for what would be allowed in a particular area and what the limitations of the system might be. This should be verified by qualified engineers.
- Conduct a Hazardous Materials Inventory, if required, to define hazard classes. This may require outside assistance to appropriately classify chemicals.
- Complete any special Hazardous Materials Permits, if required.
- Comply with the conditions of a Permit and with the technical specifications for activities such as maintenance; recognize and manage any changed conditions.
- Regularly inspect and audit the systems for compliance.
Managing hazardous materials is a part of doing business safely. Satisfying local fire marshal requirements will further help ensure that the company, the environment, and the surrounding community remain unharmed.

Comments: No Comments
Risk Management Plan (RMP) Changes: Proposed Rule
Since President Obama issued Executive Order (EO) 13650, Improving Chemical Facility Safety and Security, in August 2013, Kestrel has been following the USEPA’s efforts to carry out the EO, specifically as it relates to the Risk Management Plan (RMP) rule.
After extensive information gathering over the past two years, including issuing a Request for Information (RFI) and conducting Small Business Advocacy Review (SBAR) panels, the USEPA announced its proposed revisions to the RMP regulations on February 25, 2016.
Why RMP?
While chemicals are obviously an important part of so many aspects of our lives, improper handling and management can result in catastrophic releases that have severe and lasting impacts—loss of life, injury, property damage, community disruption.
The RMP Rule implements Section 112(r) of the Clean Air Act Amendments, and is aimed at preventing and/or reducing the severity of accidental chemical releases. RMP applies to all stationary sources with processes that contain more than a threshold quantity (TQ) of a regulated substance (based on toxicity, volatility, and flammability criteria). These sources must comply with the RMP regulations by taking defined steps to prevent accidents and by preparing and submitting an RMP to USEPA at least every five years.
Despite the RMP Rule, according to the February 25 USEPA press release referenced above, “While numerous chemical plants are operated safely, in the last 10 years more than 1,500 accidents were reported by RMP facilities. These accidents are responsible for causing nearly 60 deaths, some 17,000 people being injured or seeking medical treatment, almost 500,000 people being evacuated or sheltered-in-place, and costing more than $2 billion in property damages.”
These impacts—amongst other things—reinforce the EO and highlight the importance of modernizing the existing RMP Rule to:
- Improve chemical process safety
- Assist authorities in planning for and responding to accidents
- Improve public awareness of chemical hazards at regulated sources
Proposed Rule
The proposed amendments, as outlined in the table below, are intended to improve the requirements to enhance chemical safety at RMP facilities. Of important note, the USEPA is not proposing any revisions to the list of regulated substances under RMP at this time; however, the Agency may propose additions to this list in a separate action.
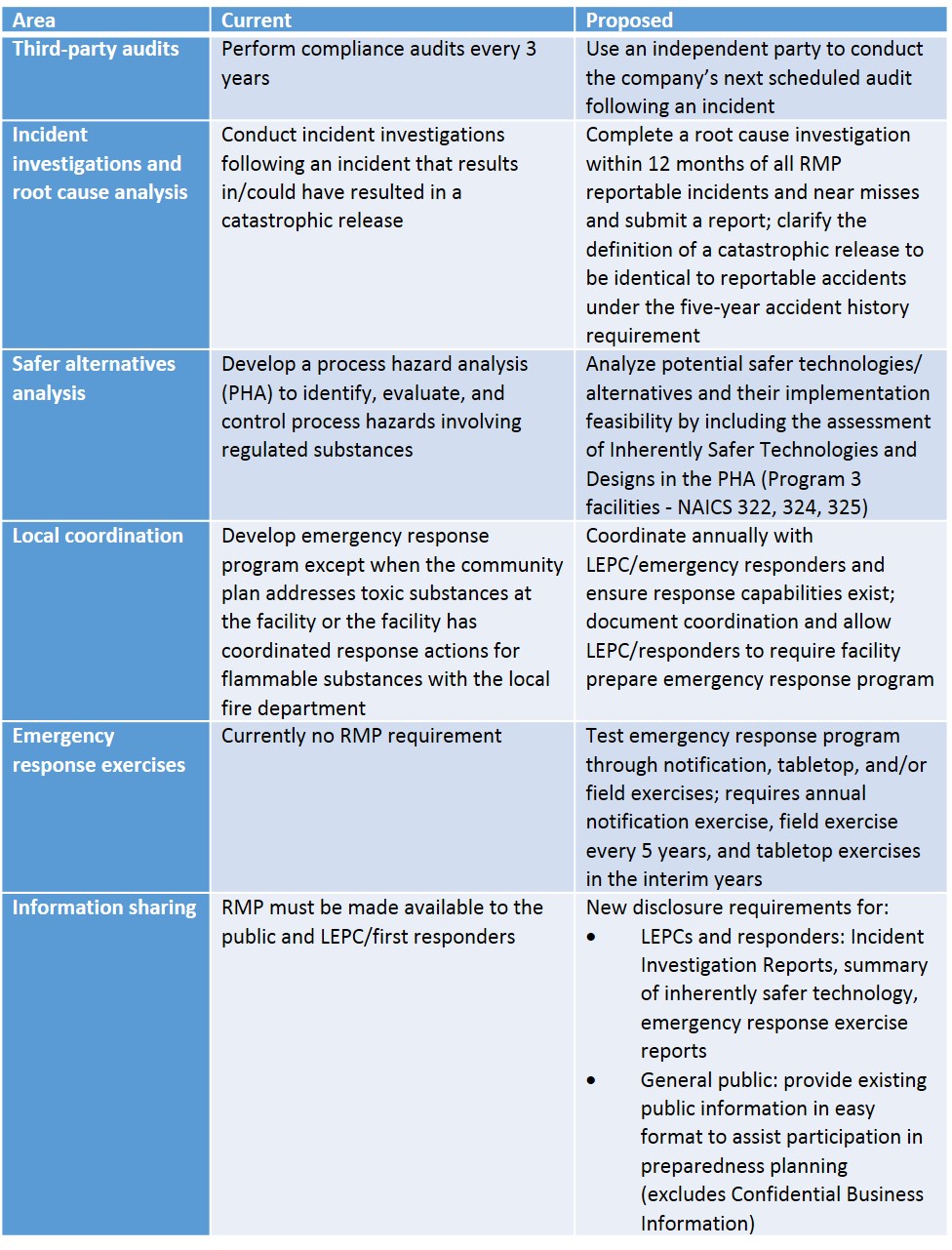
Things to Consider
There are a number of alternatives that the USEPA is still considering the proposed changes outlined above. The Agency plans to hold a public meeting to allow stakeholders to comment on the proposed rule; written comments may also be submitted within 60 days after the proposed rule is published in the Federal Register.
In reviewing and commenting on the proposed rule, it is important to consider the following:
- How might the proposed amendments impact your business?
- What additional and/or different criteria for third-party auditors should be required?
- What clarification may be required to effectively coordinate with LEPCs?
- What information is appropriate to share to improve emergency coordination with local responders and the community?
- What issues does your facility foresee with rule compliance?
Again, the proposed changes to the RMP Rule represent just one of the actions that the U.S. government is undertaking to improve chemical safety and security. Kestrel will continue to track these amendments, as well as other actions and decisions that may impact chemical facility operations.
