
KTL to Present at the Food + Beverage Environmental Conference
The Food + Beverage Environmental Conference (FBEC) is the premier and most comprehensive environmental event for the food and beverage industry in the U.S., focusing on the latest trends and innovations affecting sustainability, water resource management, supply chain, air quality, environmental compliance, and much more.
When: March 31 – April 3, 2025
Where: Rennaissance Downtown Phoenix
Who: Environmental professionasl in any business related to the food and beverage industry
Poster Presentation
Be sure to stop by and see the KTL and Ventura Foods poster at the Poster Session & Social Hour on Thursday, April 3 at 5:00 pm: Using Existing Software to Build the Ventura Foods EMS. We will talking about how Ventura Foods built and implemented an ISO 14001 EMS to meet customer requirements with limited resources.

Comments: No Comments
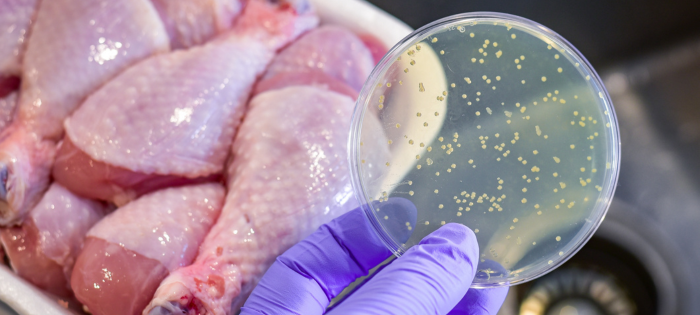
Preventing Foodborne Illness and Avoiding Recalls
The U.S. experienced several high-profile food recalls in 2024 that have caused illnesses and deaths—and heightened public awareness and unease. The emergence of new bacterial and viral strains and the evolution of pathogens that are becoming resistant to traditional food safety measures are growing concerns when it comes to preventing foodborne illnesses. According to the U.S. Public Interest Research Group’s Food for Thought 2025 Report, hospitalizations and deaths from contaminated food doubled in 2024, and recalls from Listeria, E. coli, and Salmonella increased by 41%.
Regulatory Focus on Microbiological Safety
The Food and Drug Administration’s (FDA) new unified Human Foods Program outlines a number of strategies focused on microbiological food safety that are intended to prevent and mitigate foodborne illnesses. In January 2025, FDA also released the draft Establishing Sanitation Programs for Low-Moisture Ready-to-Eat (LMRTE) Human Foods and Taking Corrective Actions Following a Pathogen Contamination Event: Guidance for Industry to help manufacturers of LMRTE human foods comply with 21 CFR part 117 and establish routine sanitation programs that can prevent pathogen contamination events.
FDA’s LMRTE Guidance specifically discusses the importance of establishing and implementing a detailed Sanitation Program and routine environmental monitoring to help prevent pathogen contamination events.
Sanitation and Good Manufacturing Practices (GMPs)
Having a properly implemented Sanitation Program and adequate GMPs provides the essential foundation for preventing pathogen harborage and product contamination events.
GMPs are intended to reduce cross-contamination by defining hygienic personnel practices, controlling traffic and product flow, ensuring proper equipment design and maintenance, and ensuring sanitary building conditions. To be effective, GMPs must be developed based on the company’s specific operations and must be implemented consistently to minimize the risk of contamination and foodborne illness.
Correspondingly, Sanitation Programs include written procedures outlining how cleaning and sanitizing tasks should be done, including the frequency and methods for cleaning surfaces, equipment, and utensils. Sanitation Programs should be regularly monitored, which might include visual checks/inspections for cleanliness. Lastly, Sanitation Programs need to be verified for effectiveness, which brings in environmental monitoring.
Environmental Monitoring
From a consumer protection perspective, environmental monitoring is intended to protect consumers by keeping harmful pathogens from contaminating the food we eat. An Environmental Monitoring Program (EMP) refers to an entire program that focuses on identifying, monitoring, and mitigating environmental risks, particularly pathogens—and foodborne illness—that may compromise food safety. EMPs involve systematic sampling and testing of the production environment for pathogens, spoilage and indicator organisms, and allergens to verify that the Sanitation Program and associated controls are working as intended to prevent foodborne illness.
Environmental monitoring is typically done by swabbing various surfaces in a designated bio-zone for pathogens and sending those samples to an accredited lab for analysis. This monitoring helps to:
- Assess how effective the plant’s cleaning, sanitation, and cross-contamination processes are.
- Determine whether there are any pathogen harborage points in the facility so it can respond accordingly (e.g., adjusting cleaning procedures, addressing equipment or facility issues, etc.).
Ultimately, the purpose of an effective EMP is to help a facility identify and implement strategies to eliminate pathogens and prevent their recurrence. It can help serve as an early warning system for identifying a potential contaminant before it spreads throughout the facility and into food that reaches the consumer. Analyzing EMP data collected over time for trends is particularly effective in continuously improving sanitary conditions.
To-Do Today
There are a number of key actions food processors can undertake today to prevent environmental pathogens from contaminating their facilities and food products:
- Apply GMPs. GMPs apply to EMPs, as with every other aspect of a strong Food Safety Program. GMPs that will impact environmental monitoring results include employee hygiene practices, cross-contamination controls, sanitary facility and equipment design, and cleaning and sanitation processes.
- Evaluate, implement, and verify preventive controls. Identify your greatest risks for environmental pathogens and proactively develop strategies to implement controls in your process flow. These may include controlling pedestrian walkways to avoid personnel contamination; using dedicated tools, equipment, and/or staff post-process; having special uniforms for staff, etc. Just as important, you must verify the performance of your preventive controls through environmental monitoring and take corrective action immediately if problems arise.
- Ensure employees and other resources are qualified. Employees responsible for sanitation, sampling, and overseeing the EMP must have the necessary training and/or experience for assigned duties. In addition, any outside labs used for environmental testing must be accredited under ISO 17025.
- Regularly review and update the Sanitation Program, GMPs, and the EMP. Products, operations, equipment, employees, processes, and other environmental factors change—and all of these can impact the Sanitation Program, GMPs, and the EMP. Conducting periodic reviews to ensure processes and procedures reflect any new conditions is important in ensuring a facility’s overall hygiene and its products’ quality and safety. Reviewing the EMP results and trending on a regular basis and adjusting your program according to the results is important to keep ahead of any potential issues.

Comments: No Comments
Food Safety: Top Trends for 2025
The food system and supply chains continue to change and evolve at an accelerated pace. That introduces new risks and challenges—and heightens some familiar ones. As we move into 2025, the Food and Drug Administration (FDA) has established priorities that will impact how food companies operate in the coming year. In addition to the FDA’s priorities, here are some of the top food safety trends KTL’s food safety experts are tracking in 2025…
FDA 2025 Priorities
On October 1, 2024, the Food and Drug Administration (FDA) began implementing its reorganization, including launching a unified Human Foods Program (HFP) to oversee all activities related to food safety and nutrition. The HFP has prioritized key deliverables for FY 2025 to strengthen regulatory oversight, advance science, and better leverage partnerships in the areas of microbiological food safety, food chemical safety, and nutrition. The FDA also restructured the Office of Regulatory Affairs (ORA) into the Office of Inspections and Investigations (OII) to allow the Agency’s field operations unit to focus on inspections, investigations, and imports.
As with any regulatory change, FDA-regulated companies need to understand the restructuring and its potential impacts. The HFP has outlined priorities and compliance expectations that companies need to align with their internal programs, protocols, etc. It will also be important to prepare for potential changes when it comes to inspections, including more frequent and more specialized inspections.
Update: According to Food Safety Magazine, on January 21, 2025, federal public health agencies within the U.S. Department of Health and Human Services (HHS) received orders from the new Administration to temporarily pause all external communications until further notice. This includes the FDA and U.S. Center for Disease Control and Prevention (CDC), which are jointly responsible for ensuring the safety of most of the U.S. food supply and responding to foodborne illness outbreaks. All external communications must be reviewed and approved by a Presidential appointee before being issued. The directive will be in effect until February 1, 2025
Foodborne Illness
The U.S. has experienced several high-profile food recalls in 2024 that have caused illnesses and deaths—and heightened public awareness and concern. The emergence of new bacterial and viral strains and the evolution of pathogens (e.g., Listeria, E. coli, Salmonella) that are becoming resistant to traditional food safety measures are growing concerns when it comes to preventing foodborne illnesses.
The FDA’s focus on microbiological food safety (see above) is intended to advance strategies to prevent and mitigate foodborne illnesses. On July 29, 2024, U.S. Department of Agriculture (USDA) issued its proposed rule and determination to more effectively reduce Salmonella contamination and illnesses associated with raw poultry products; final rulemaking is anticipated in 2025. The USDA Food Safety and Inspection Service (FSIS) further announced several immediate initiatives to enhance its approach to mitigating foodborne pathogens, including modernizing L. monocytogenes regulation and ready-to-eat (RTE) sampling, in response to the Boar’s Head inspection results.
Companies need to re-evaluate their food safety programs and their effectiveness. This review may necessitate changing the current level of scrutiny for current and prospective suppliers. If sourcing any products implicated in recalls, re-evaluating current suppliers’ compliance with food safety regulations is also recommended. Sanitary equipment design, robust cleaning procedures, effective traceability programs, adherence to Good Manufacturing Practices (GMPs), and adequate training will remain key to avoiding recalls.
Supply Chain Traceability
There continues to be a real need for standardization, stronger linkages throughout the supply chain, improved communication and recordkeeping, and faster response when it comes to product traceability. As such, the FDA has prioritized the advancement of traceability tools and resources to help remove contaminated products from the marketplace more quickly.
From a regulatory standpoint, companies must start meeting the requirements of the Food Traceability Rule by January 2026, though there is speculation this may be delayed due to a provision in the current draft House appropriations bill that would prohibit funding for implementation, administration or enforcement of these regulations. Regardless, organizations should be performing traceability exercises to help identify gaps, testing protocols and verifying effectiveness, implementing corrective actions, and ensuring adequate traceability processes are in place. Investing in a good technology solution that integrates with the food safety management system (FSMS) will help to further streamline the process.
Sustainability
The U.S. Environmental Protection Agency (EPA) cites that food and food packaging materials make up almost half of all municipal solid waste in the U.S. Federal and state regulatory agencies, as well as various food safety certification schemes, have begun incenting—or requiring—organizations to incorporate sustainable food management and recycling practices into their operations. For example, the New Jersey Recycled Content Law, which came into effect on January 18, 2024, is considered one of the most ambitious recycled content laws to date.
A thorough food and packaging assessment serves as the foundation for reduction efforts. Having a general understanding of operations and waste streams can help identify appropriate strategies to avoid waste, cut down on disposal costs, reduce over-purchasing and labor costs, optimize inventory management, use more recycled content, and reduce water and energy use associated with food production.
Employee Safety
Between October 2018 and September 2019, the Occupational Safety and Health Administration (OSHA) issued 1,168 citations resulting in over $7 million in fines to the food manufacturing industry, with lockout/tagout and machine guarding topping OSHA’s annual list of the most frequently cited standards in food manufacturing. Occupational safety and health risks in food manufacturing are often heightened because of the nature of the product (i.e., food or drink) being manufactured.
OSHA is watching, and facilities need to prioritize employee safety. Conduct a thorough hazard analysis of the facility, operations, and processes to identify potential safety hazards. Based on the analysis, develop, implement, and maintain the appropriate safety programs, procedures, and instructions. Provide the proper personal protective equipment (PPE) to keep employees safe. And, importantly, train employees so they understand what they need to do to protect themselves and others.
PFAS
The EPA continues to make significant contributions to confront the human health and environmental risks of per- and polyfluoroalkyl substances (PFAS). In April 2024, EPA introduced the first national drinking water standards for PFAS, and many states are introducing policies related to PFAS, including monitoring and testing for PFAS in water and banning PFAS in food packaging, clothing, and other consumer products. The FDA is further working to limit PFAS in food and food packaging. In 2024-2025, the FDA has committed to testing foods from the general food supply to more accurately estimate exposure to PFAS from foods. This includes testing total dissolved solids (TDS) samples, conducting a survey of bottled water, and conducting additional seafood testing.
Organizations need to address PFAS in their operations, products, supply chains, and waste streams; stay informed about ongoing PFAS regulatory developments; and adjust compliance programs and operational practices, as needed.
Digital Transformation
According to McKinsey & Company, automation could increase productivity by up to 20% in the food manufacturing sector. More companies are employing technology solutions to improve business efficiency, and the FDA has prioritized developing AI approaches to help monitor new data and trends in FY 2025.
AI is quickly becoming a more viable solution for many food companies in automating, monitoring, and managing food safety, helping to identify contamination risks, analyze data, predict potential hazards, conduct training simulations, and even optimize cleaning schedules. While the efficiencies and other benefits of technology solutions are many, the increased reliance on AI will require an investment, not just financially but also in oversight and management, training, and cybersecurity measures. As the digital transformation continues, companies will need to determine where automation is appropriate and where the human factor is still required.
Others to Watch
- Red No. 3: On January 15, 2025, the FDA amended its color additive regulations to no longer allow for the use of Red No. 3 in food and ingested drugs. Manufacturers who use Red No. 3 will have until January 15, 2027 (food) or January 18, 2028 (ingested drugs) to reformulate products. Foods imported into the U.S. must also comply with this requirement.
- Produce Safety Rule: Pre-Harvest Agricultural Water: In May, 2024, the FDA released the updated requirements for the Produce Safety Rule, Subpart E for pre-harvest agricultural water. Facilities are now able to justify not testing agricultural water if there are no conditions (e.g., animals, human waste, or biological soil amendments of animal origin (BSAAO)) on nearby or adjacent land that pose a risk to the water source. The first compliance date for large farms is April 7, 2025.
- SQFI New Edition 10: The Safe Quality Food Institute (SQFI) is slated to release SQF Edition 10 in July 2025. Anticipated updates focus on the identification of new food safety risks, changes in the global supply chain that may impact food safety, unforeseen events (e.g., foodborne illness outbreaks), new technology, and harmonization with other internal food safety programs.
- Bird Flu (H5N1): With the rise in cases and mutations, the USDA has announced different approaches to prevent the spread of H5N1, including testing bulk raw milk. The State of California also declared a state of emergency to allocate more resources to address the continued spread of Bird Flu.
- Food Date Labeling Reform: In December 2024, the FDA and USDA announced a joint Request for Information (RFI) regarding food date labeling (e.g., ‘Sell By,’ ‘Use By’ and ‘Best By’). Open date labeling is currently not mandated or standardized by federal law. The RFI seeks information on industry practices and preferences for date labeling.
Set Your Goals for 2025
In 2025, organizations will continue to encounter workplace challenges, regulatory adjustments, food safety concerns, and rapid technology advancements—and, in most cases, fewer resources to manage it all. KTL suggests completing the following early in 2025 to prepare for whatever is on the horizon:
- Get senior leadership commitment and invest in creating a food safety culture that prioritizes food safety, quality, and employee health and safety. Focus on changing from a reactionary to a preventive mindset. And remember that your people are as important as your products.
- Conduct a comprehensive food safety and quality gap assessment. Know your operations, inventory your ingredients, understand your supply chain, quantify your food waste. This assessment should be the starting point for understanding your regulatory and certification obligations and current compliance status—and for ensuring you are prepared to meet pending regulatory developments.
- Explore technological advancements that allow for further digitization and promote more timely and accurate collection and management of important data. This could mean stepping into the world of AI and machine learning; it could also mean implementing a digital FSMS to help manage compliance/certification and recordkeeping requirements. Technology advancements can help create significant business efficiencies when used appropriately.
- Train your staff. Every trend discussed above requires employee understanding. Train your team routinely on requirements, responsibilities, processes, expectations, etc. Of particular note for Preventive Controls Qualified Individuals (PCQIs), the International Food Protection Training Institute (IFPTI) has released Version 2.0 of the Preventive Controls for Human Foods course. The old version will no longer be taught as of May 2025, but the training will still be recognized.
- Seek third-party oversight. Having external experts periodically look inside your company provides an objective view of what is really going on, helps you to prepare for audits, and allows you to implement corrective/preventive actions that ensure compliance. An outside expert can often provide the “big picture” view of what you have vs. what you need; how your plans, programs, and requirements intersect; and how you can best comply with changing requirements.

Comments: No Comments
FDA Restructuring: Impacts on Industry
On October 1, 2024, the Food and Drug Administration (FDA) began implementing the largest reorganization in the Agency’s recent history, including launching a unified Human Foods Program (HFP) to oversee all activities related to food safety and nutrition and transforming the Office of Regulatory Affairs (ORA) into the Office of Inspections and Investigations (OII), amongst other modernizations. This reorganization is intended to enhance the FDA’s ability to protect the human food supply and make the Agency more efficient and prepared to respond to changes in industries, food and medical product technologies, and the impacts of globalization and climate change.
HFP: Priorities for 2025
The FDA regulates 80% of the U.S. food supply. The HFP was designed to help ensure that the FDA-regulated food supply is safe by taking a systematic, risk management approach to implementing the preventive measures outlined in the Food Safety Modernization Act (FSMA) and responding to food-related emergencies. Specifically, the HFP’s mission is to protect and promote the health and wellness of all people through science-based approaches to prevent foodborne illness, reduce diet-related chronic disease, and ensure chemicals in food are safe.
To support this mission, FDA is implementing several operational changes, including developing enhanced risk modeling, advancing inspectorate training, establishing a Human Foods Advisory Committee, developing a performance management framework, improving recall communications, and integrating food laboratories to advance science.
The HFP has also prioritized key deliverables for FY2025 to strengthen regulatory oversight, advance science, and better leverage partnerships in the following risk management areas:
Microbiological Food Safety
Advancing strategies to prevent foodborne illness. Specific deliverables include:
- Finalizing an implementation plan for the FSMA Final Rule on Pre-harvest Agricultural Water.
- Advancing traceability tools and resources to remove contaminated products from the marketplace more quickly.
- Issuing final guidance for the Produce Safety Rule and developing associated compliance resources.
- Initiating a study to better understand Salmonella outbreaks linked to melons.
- Enhancing the use of GenomeTrakr to help identify and respond to outbreaks.
- Working with various stakeholder groups to develop strategies to prevent and mitigate microbiological foodborne risks.
- Enhancing communication and transparency, including publishing a new public outbreak report and facilitating information sharing related to fresh produce and seafood.
- Implementing regulatory partnerships with Ecuador, India, and Indonesia for imported seafood.
Food Chemical Safety
Ensuring exposure to chemicals in food is safe. Planned actions include:
- Reviewing inefficiencies in the current premarket review process for food or color additives to help prevent unsafe uses of chemicals.
- Updating the framework for post-market assessments of chemicals in food and publishing an updated list of substances prioritized for re-assessment.
- Establishing action levels and associated guidance for environmental contaminants in food intended for infants and young children.
- Releasing additional guidance on submitting new dietary ingredient notifications (NDINs).
- Issuing draft guidance for Preventive Controls for Human Food specific to chemical hazards.
- Completing external review and validation of the Expanded Decision Tree, which sorts chemicals into classes of toxic potential.
- Developing AI approaches to help monitor new data and trends.
- Evaluating and characterizing potential effects of PFAS exposure from selected foods.
- Partnering with international organizations on food chemicals and innovation to strengthen the global food safety system.
Nutrition
Elevating nutrition to improve health equity. Deliverables include:
- Issuing a final rule to update the definition of the claim “healthy” and educating the public.
- Proposing a rule to establish mandatory front-of-package nutrition labeling.
- Publishing a long-term national strategy to help facilitate the entry of new infant formula manufacturers and continuing education on safe handling of powdered infant formula.
- Advancing nutrition research to better inform food-related policy and regulatory decision making.
OII’s Focus on Inspections
Restructuring the ORA into the new OII is intended to allow the Agency’s field operations unit to focus on inspections, investigations, and imports to support its core mission to conduct rigorous, transparent, and science-based inspections and investigations, providing real-time evidence and insight essential in empowering fact-based regulatory decisions to protect public health. OII is charged with inspecting regulated products and manufacturers, as well as reviewing imported products.
This restructuring will enable the FDA to put more focus directly on inspections and investigations and, subsequently, create a more efficient compliance and enforcement process, clearer communication, and faster decision-making. As a result of this transformation, inspections have the potential to become more efficient—and more frequent. In addition, increased inspection resources may allow inspectors to specialize in specific product areas, which could lead to more thorough and knowledgeable inspections.
Keeping Up
As with any regulatory change, FDA-regulated companies need to get up to speed on FDA’s recent restructuring and its potential impacts:
- Educate staff on FDA’s reorganization and potential implications for your organization.
- Identify any new points of contact for your organization within the FDA (i.e., HFP, OII), anticipate how your interactions may change, and start building new relationships, where necessary.
- Review the HBP priorities and compliance expectations for FY2025 compared to internal programs, protocols, etc. and make any required updates to ensure alignment.
- Prepare for potential changes when it comes to inspections, including more frequent and more specialized inspections. This may include educating staff to get a deeper understanding of products/operations and the associated regulatory requirements and/or stepping up compliance efforts, where necessary.

Comments: No Comments
Food Date Labeling Reform
“Best if Used by”, “Sell by”, “Expires on”, “Freeze by”…
Consumers see various versions of these phrases on food product labels all the time. But do they understand what these labels mean? Does industry? According to the Food and Drug Administration (FDA), it is estimated that the confusion over date labeling terms on food products accounts for about 20% of the food waste in the home. This is significant given that the U.S. Department of Agriculture (USDA) estimates nearly one-third of available food in the U.S. (i.e., 133 billion pounds) goes uneaten due to food loss or waste.
Why the confusion? Because open date labeling is still not mandated or standardized by federal law. In fact, the State of California just recently passed the first legislation of its kind in September 2024 to standardize the use of open date labeling on food products.
Nutrition Labeling vs. Open Date Labeling
The Nutrition Labeling and Education Act (NLEA) of 1990 requires that food manufacturers provide standardized nutritional information on their products. It also requires that food labels comply with certain requirements if they make nutritional or health claims. The FDA developed the Nutrition Facts Label to comply with the requirements of the NLEA.
The phrases mentioned above are considered open date labeling and are not part of the NLEA. Any open date labeling on food products is applied voluntarily by manufacturers or retailers to tell consumers when food is of best quality. With the exception of infant formula, these dates are not required and are not related to a product’s safety.
The USDA gives the following definitions of common phrases on open date labels:
- “Best if Used by/before” indicates when a product will be of best flavor or quality. It is not a purchase or safety date.
- “Sell by” tells the store how long to display the product for sale. This phase is for inventory management; it is not a safety date.
- “Use by” provides the latest date recommended for the use of the product while at peak quality. It is not a safety date (except on infant formula).
- “Freeze by” indicates when a product should be frozen to maintain peak quality. It is not a purchase or safety date.
A Question of Quality
For most, the confusion lies in understanding the status of a food product once the open date passes. While the quality of perishable products may deteriorate after a “Best if Used by” date, most products remain safe to eat, sell, or donate as long as they are handled properly (e.g., correct temperature and storage conditions) and show no signs of spoilage. In fact, the USDA suggests that food handling conditions are a greater predictor of spoilage than time (i.e., open date).
Sources including FDA, USDA, and a number if industry associations believe that food waste is due, in part, to fears about food safety and consumers not understanding that date labels refer to the quality of a product versus its safety to consume.
Call for Standardization
Despite this confusion and the associated ramifications (i.e., wasted food, economic loss), no mandatory federal labeling standard has been enacted, though attempts at standardization have been made:
- The Food Recovery Act of 2015 introduced the first language to standardize date labels, but it did not pass Congress.
- In December 2016, the USDA Food Safety and Inspection Service (FSIS) updated its guidance on product date labeling, recommending that manufacturers and retailers use the phrase “Best if Used by” to indicate when a product is at peak quality.
- In 2017, industry stepped in, as the Food Manufacturing Institute (FMI) and the Consumer Brands Association (CBA) adopted joint industry guidelines to standardize open dates.
- The FDA issued a letter supporting industry’s efforts to standardize date labeling in May 2019. Consistent with the USDA, the FDA recommended using the “Best if Used by” date for manufacturers choosing to apply a quality date label.
- In 2019, the first Food Date Labeling Act (FDLA) was introduced in Congress and failed to pass. The FDLA was introduced again in 2021 and, most recently, in 2023.
California Leads the Way
In the meantime, the State of California has forged ahead, passing legislation in September 2024 to standardize the use of open date labeling on food products. Beginning July 1, 2026, the law implements the first mandatory food date labeling for companies selling food products in California by instituting the following changes:
- “Best if Used by” must be used to indicate the date when a product reaches peak quality.
- “Use by” must be used to indicate the date when a product’s safety can no longer be guaranteed.
- Retailers will be authorized to display “Packed on” labels on prepared food items, but they must also display the “Best by” or “Best if Used by” dates.
- Consumer-facing “Sell by” dates are prohibited and may only be used for inventory management.
- The phrases “Expires on” and “Freshest by”, which have inconsistent definitions, are prohibited.
Support from Industry
According to the Zero Waste Food Coalition, over 25 industry supporters have signed the Zero Food Waste Coalition’s open letter urging Congress to support the bipartisan Food Date Labeling Act of 2023… Major food industry businesses, including Kroger, Walmart, and Nestlé USA, have signed the letter, which cites that standardizing and streamlining date labels is one of the most cost-effective methods to prevent the wasting of surplus, wholesome food.
Currently, it is at the food manufacturer’s/retailer’s discretion to use open date labeling as they see fit. As such, it is important for manufacturers and retailers to understand what the various open date labeling phrases mean before applying them to products. The available USDA, FDA, and industry guidance is meant to alleviate consumer confusion and should be used as best practice in absence of a federal standard. Using this guidance to standardize open date labeling practices to the extent possible may help manufacturers extend the shelf-lives of their products, prevent the corresponding economic loss of removing inventory from the supply chain, and reduce the amount of wholesome food being wasted.

Now Hiring: Safety Consultant
Location: Chicago, Illinois or St. Lous, Missouri
KTL is seeking an experienced Safety Consultant based in the Chicago, IL or St. Louis, MO metropolitan area with a strong safety compliance background working as a consultant or in industry to join our team. This individual will have knowledge of general industry safety procedures and Occupational Safety and Health (OSHA) requirements. The Safety Consultant will have experience auditing, developing, and implementing OSHA safety programs in an industrial setting, and expertise helping organizations proactively manage their operational safety-related risks.
Responsibilities and tasks include the following:
- Conducting OSHA compliance audits in general industrial settings.
- Researching federal, state, and local regulatory requirements and helping maintain standards.
- Effectively managing and building client relationships, leading to repeat business.
- Developing and delivering training and coaching on safety.
- Performing site safety assessments, conducting incident investigation/root cause analysis, and implementing follow-up corrective actions.
- Working with a team to assess, design, implement, and audit safety management systems and related programs.
- Designing safety performance metrics to drive continual improvement.
- Applying quality and process improvement methods and tools.
- Writing technical reports, work plans, and proposals.
- Supporting other KTL professionals to effectively manage and deliver projects.
- Managing tasks remotely and at client sites.
Requirements
- B.S. degree in Environmental Science, Engineering, or Occupational Safety preferred.
- 5-10 years of consulting or relevant experience working in safety for industry/manufacturing.
- Associate Safety Professional (ASP) or Certified Safety Professional (CSP) preferred.
- Recent experience conducting safety audits.
- Strong knowledge of OSHA safety standards and programs.
- Experience with environmental compliance preferred.
- Experience in healthcare preferred.
- Previous project management experience and ability to manage multiple projects and clients.
- Excellent verbal and written communication, interpersonal, and presentation skills.
- Excellent research, analytical, writing, and organizational skills.
- Ability to work independently and as a part of a team.
- Proficient in Microsoft 365 software.
- Valid driver’s license.
- Ability to travel up to 50%.
How to Apply
Forward a resume to recruiting@goktl.com.
Company Description
KTL is a multidisciplinary consulting firm that specializes in providing environmental, health, and safety (EHS); food safety; and quality management and compliance consulting services to industry and government clients. Our primary focus is to build strong, long-term client partnerships and provide tailored solutions to address regulatory compliance requirements. KTL’s services include auditing and assessments, management system development and implementation, certification support, regulatory compliance assistance, information management solutions, and training. Our headquarters are in Madison, WI and Atlanta, GA, with satellite offices throughout the U.S.

Don’t Miss KTL at the 2024 Food Safety Consortium
KTL is excited to be joining the 2024 Food Safety Consortium in Washington, DC, October 20-24, 2024. The 13th Annual Food Safety Consortium provides food safety and quality assurance professionals with cutting-edge knowledge, practical skills, and a collaborative network to enhance their professional development as champions of food safety.
KTL will be leading the following breakout session as part of the workshop’s technical agenda:
The Big Secret…You already have the software you need to manage food safety
October 22, 2024 | 8:30-9:15 am | Presenter: Roberto Bellavia, Senior Consultant and Partner
This session will demonstrate how food companies are leveraging Microsoft Power Platform with SharePoint® to elevate the food safety management systems (FSMS) and more effectively manage food safety compliance documentation, data, and certification requirements.
And be sure to stop by and visit us at Booth #11. We look forward to seeing you at the Food Safety Consortium!
Comments: No Comments
Stopping Salmonella: Proposed USDA Framework
Salmonellosis (i.e., an infection with the bacteria Salmonella) is the second leading cause of foodborne illness in the U.S. The (CDC) Center for Disease Control estimates over 1.35 million illnesses, 26,500 hospitalizations, and nearly 420 deaths annually are attributed to Salmonella. It is estimated that over 23% of those illnesses are from eating chicken or turkey.
On October 19, 2021, the U.S. Department of Agriculture (USDA) launched a comprehensive effort to reduce Salmonella illnesses associated with poultry products. Since then, the Department has completed several activities to move closer to the national target of a 25% reduction in Salmonella illnesses (see below). One of the most significant actions came on July 29, 2024, when the USDA issued its proposed rule and determination to more effectively reduce Salmonella contamination and illnesses associated with raw poultry products.
Salmonella 101
Salmonella are bacteria that live in the intestinal tract of humans and animals and can contaminate some types of food, including raw fruits and vegetables and raw or undercooked chicken, turkey, beef, and eggs. Salmonella is transmitted by consuming contaminated food products. It can also be transferred when handling pets, particularly birds and reptiles.
Controlling Salmonella contamination is tricky for several reasons. There are approximately 2,500 serotypes (i.e., distinct strains) of Salmonella bacteria, and their risks differ across all production systems. Salmonella can survive under extreme conditions and adapt to its environment. It can also survive in most animals without causing disease in the animal. In fact, Salmonella can be found just about anywhere, including in food, water, soil, and the air we breathe.
Regulatory History
USDA’s most recent proposed rule and determination represents the culmination of the Food Safety and Inspection Service’s (FSIS) three-year effort to better control Salmonella rates in poultry and protect American consumers. The following significant events have led to this latest action:
- October 2022: FSIS announces a draft regulatory framework for a new strategy to control Salmonella in poultry products.
- June 2023: The USDA Agricultural Research Service (ARS) launches the Salmonella Grand Challenge, bringing together a group of scientists from different specialties to learn more about how and where Salmonella causes the highest risk to meat and poultry products.
- April 2024: FSIS publishes a final policy to declare Salmonella an adulterant in raw breaded chicken products when they exceed a threshold of 1 colony forming unit (CFU) per gram of Salmonella contamination. This represents the first time the USDA has labeled Salmonella as a contaminating adulterant in food, alongside certain types of E.coli.
Requirements of the Proposal Rule
The July 2024 proposed framework presents a systematic approach to addressing Salmonella contamination at the poultry slaughter and processing stages. It includes the first enforceable standards for Salmonella, something that has not been established until now because previously available tools and technology were not sufficient to track the bacteria.
The rule can be broken into the following two parts:
Enforceable Limits
The proposed rule establishes final standards to prevent raw chicken carcasses, chicken parts, ground chicken, and ground turkey products that contain any type of Salmonella at or above 10 CFU per gram/mL from entering the market. It also requires facilities to test for the presence of the following, which have been deemed Salmonella serotypes of public health significance:
| Product | Serotypes* |
| Chicken carcasses, chicken parts, comminuted (ground) chicken | – Enteritidis – Typhimurium – I4,[5],12:i |
| Comminuted (ground) turkey | – Hadar – Typhimurium – Muenchen |
If the bacteria exceed the established threshold of 10 CFU per gram/mL and contain any detectable level of at least one of the identified serotypes, the poultry cannot be sold, and the product lot would be subject to recall.
Monitoring, Sampling, and Testing
The final rule also requires poultry companies to establish microbial monitoring programs (MMPs) to identify and prevent pathogen contamination throughout the slaughter system. All poultry slaughter establishments would be required to develop, implement, and maintain written procedures to prevent contamination and maintain records documenting those procedures. More specifically, they must incorporate statistical process control (SPC) monitoring principles into their MMPs to monitor the quality of the manufacturing process. Facilities must also implement written corrective actions, including root cause analysis, when MMP results differ from the predefined criteria.
Finally, the proposed standard provides for a routine sampling and verification testing program for Salmonella in chicken parts, comminuted chicken, and comminuted turkey. FSIS would sample raw final products and analyze them for Salmonella levels and serotypes to determine whether the final product is adulterated. If test results detect Salmonella at a level of 10 CFU/mL or higher and at least one Salmonella serotype of public health significance, FSIS would consider products to be adulterated and the lot would be prohibited from entering commerce or a recall would be initiated.
Moving Forward
Setting limits on Salmonella levels has been difficult until now, because existing tools and technology were not sophisticated enough to track the bacteria. USDA Undersecretary for Food Safety Dr. Emilio Esteban has stated of this proposed rule, “It’s time to change our approach…We have the tools. We have the technology. We have the knowledge.”
The USDA also has the track record. The Department took similar action with E.coli in 1994 after deadly outbreaks tied to ground beef. As a result, the number of foodborne illnesses related to E.coli has fallen by more than 50%. The ultimate goal of the framework for Salmonella is similar—to get the U.S. closer to the national Healthy People 2030 target of a 25% reduction in salmonellosis cases.
USDA is accepting comments on the proposed final regulatory framework through September 29, 2024.

Comments: No Comments
Food Chemical Safety
Safety Focus
Food chemical safety is an area that is becoming a growing public concern, especially with states including California, Illinois, and New York challenging the safety of certain food additives and other chemicals used in food. Is food safe to eat if it has chemicals?
Chemical Presence in Food
The truth is…all our food is made up of chemicals. Some naturally exist in whole foods and provide nutrition. For example, the potassium in bananas is a chemical. Some chemicals, like environmental contaminants, get into food when crops absorb them from soil, water, or air. Process contaminants (e.g., undesired chemical byproducts) can also form during food processing, particularly when heating, drying, or fermenting foods.
Chemicals may also be added to food for a variety of reasons:
- Create additional nutritional benefits (e.g., vitamins A and D being added to milk).
- Provide protection from pathogens that could make people sick.
- Enhance food by adding flavor, improving texture, and changing appearance.
- Preserve quality by preventing spoilage or extending shelf life.
FDA Authorization
The Food and Drug Administration (FDA) must authorize any chemical added to food for use as a food or color additive before it may be used, unless the substance is generally recognized as safe (GRAS). Through its pre-market review programs, FDA reviews all relevant information about the chemical before providing its authorization, including information about:
- The identity of the chemical, including its chemical structure and data on other similar substances.
- How the substance will be used, its level of use, and the amount people may be exposed to in food.
- Toxicology, safety data, and other information to show the substance is safe at calculated exposure levels.
How Much Is Too Much?
The presence of a chemical does not determine whether a food is safe to eat. Rather, it is the amount that counts.
FDA scientifically assesses the safe amount of a chemical in food by comparing how much chemical is in the food and how much someone is likely to consume daily with other safety data to determine whether a food is safe to eat. Any chemical has the potential to be harmful at a certain level, which is why this multi-pronged evaluation and extensive calculations are important.
FDA determines an Acceptable Daily Intake level for the chemical. This level has a “built-in” safety margin to ensure the allowable daily amount is actually much lower than the level known to have a possible adverse health effect.
When Food Chemicals Become Unsafe
Authorized chemicals normally used in foods as additives and preservatives can become hazardous when they are unintentionally added or are present beyond the established limits. When this happens, it can cause immediate illness and/or long-term health effects on consumers. FDA monitors the food supply for chemical contaminants and takes action when the level of a contaminant causes a food to be unsafe. Situations such as this result in food safety alerts, recalls, and withdrawals from the market.
FDA helps safeguard the food supply by evaluating the use of chemicals as food ingredients and substances that come into contact with food (e.g., packaging, storing, handling). But ultimately, food manufacturers are responsible for marketing safe foods and ensuring that they meet FDA requirements. The manufacturers are required to implement preventive controls to significantly minimize or prevent exposure to chemicals in foods that may be hazardous to human health.
More information can be found on food chemical safety at the following websites:

World Brewing Congress 2024: Don’t Miss KTL on the Food Safety Panel
KTL is excited to be participating in the 2024 World Brewing Congress (WBC) in Minneapolis, MN, August 17-20, 2024. WBC 2024 aims to provide a compass for the brewing community to chart its course through the challenges that brewers and brewing professionals encounter on a global scale.
KTL will be a panelist for the following session:
Food Safety: Requirements, Programs, Experiences & Culture in Brewing
August 20, 2024 | 10:00-11:15 am | KTL Panelist: Estefania Lopez
Beer is food. This session will introduce you to basic food safety programs and regulatory requirements needed in order to produce a safe and quality product that your customers will keep coming back to and enjoy time after time.
