
Comments: No Comments
Creating Sustainable Impacts Part 2: Lifecycle Analysis (LCA)
As discussed in Part 1 of KTL’s series on Creating Sustainable Impacts, sustainable materials management (SMM) broadens the ideas behind integrated waste management (IWM) to examine all the environmental impacts of material production and consumption, not just waste diversion or recyclability. It considers the entire lifecycle (i.e., extracting, manufacturing, distributing, using, and end-of-life management) of a product and/or process. Adopting sustainable materials management (SMM), organizations can improve their triple bottom line (TBL)—reducing their environmental impacts significantly, while still increasing profit—and contribute to the overall sustainability of our world.
Analyzing the Entire Lifecycle
These SMM solutions are most effectively identified through a lifecycle analysis (LCA). As the name implies, an LCA considers potential environmental impacts at every stage of a product’s life. An LCA can demonstrate that seemingly obvious solutions are not always the best solutions. For example, non-recyclable packaging may actually have fewer environmental impacts than recyclable packaging if it is lighter and occupies less space. Understandably, solutions like this can seem counterintuitive to waste management professionals, but this example demonstrates the importance of considering the impacts of a material across its entire lifecycle.
LCAs do not replace the basic principles underlying EPA’s Waste Management Hierarchy, especially the importance of source reduction and waste prevention. In fact, LCAs generally show that most of a product’s environmental impacts occur earlier in its lifecycle (i.e., upstream) vs, at the end of its life (i.e., downstream). Thus, choosing a different raw material—or finding ways to use less—is often more impactful than end-of-life waste management solutions.
But as LCAs will show, even this concept of reducing material use is not a given for all products. For example, food packaging is vital in reducing food spoilage and subsequent wasted food. Reducing or eliminating packaging may save material, but in the end, this may lead to more wasted food and even greater environmental impacts.
As consumer goods and related packaging get more complex, an LCA considers the most effective management for materials, including how they are used, potentially reused, and eventually discarded. This ultimately helps organizations identify environmental sustainability priorities; move past one-dimensional waste management goals; and then design, select, and manage products accordingly.
Conducting an LCA
LCAs identify and quantify inputs and outputs in a process and use data to assess the potential environmental impacts across the lifecycle. According to the Sustainable Materials Management Coalition, this allows more informed decisions that:
- Evaluate environmental consequences of a given product.
- Analyze the environmental tradeoffs associated with one or more specific products/processes.
- Quantify environmental releases to air, water, and land in relation to each lifecycle stage.
- Compare the potential environmental impacts between two or more products/processes.
- Identify potential impacts to one or more specific environmental areas of concern.
- Provide a comprehensive view of the environmental aspects of the product or process and a more accurate picture of the true environmental tradeoffs in process and product selection.
ISO 14040 defines the principles and frameworks to adequately conduct an LCA, while ISO 14044 specifies the related requirements and guidelines. An ISO LCA is conducted in the following four stages:
- Goal and Scope: What do we want to measure (i.e., product/company/service)? The LCA objectives, scope, and boundaries need to be carefully selected and clearly framed.
- Lifecycle Inventory: What data do we need? Collect all the inputs and processes to be measured (i.e., raw materials, energy used/purchased, supplier data). The inventory data is used to assess the energy, water, and materials used, as well as identified environmental releases.
- Impact Assessment: What is the impact of the lifecycle inventory? Impact assessments take the results of inventories and convert them into more easily understood impact categories, such as global warming potential or carcinogenic potential.
- Interpretation: What does this all mean? (i.e., How high are our emissions? How do our products compare? Can we improve them? Can we improve our processes? What are the biggest levers for us?)
While not all LCAs need to follow the rigors of these ISO standards, it is useful to incorporate lifecycle thinking such as this into SMM decision-making. In some cases, it might be as simple as considering the potential environmental ramifications of major steps in the value chain. Adopting this lifecycle perspective will help to provide a clearer understanding of the environmental implications of everyday choices.
Part 3 of our series on Creating Sustainable Impacts dives into one of the largest opportunities for SMM — wasted food.

Comments: No Comments
National Incinerator Slowdown
According to Environmental Protection, more than 200 million tons of hazardous waste are generated each year. Much of that hazardous waste is destroyed in permitted, regulated incinerators located throughout the U.S. These incinerators are heavily monitored and have robust emissions management systems in place. In fact, the U.S. Environmental Protection Agency (EPA) considers hazardous waste incineration to be the Best Demonstrated Available Technology (BDAT) for most organic hazardous waste because of how safely and effectively hazardous constituents are destroyed and waste is converted into ash, flue gas, and heat. Frequently, these facilities also have energy recovery systems that capture BTU value from the incinerated waste, resulting in peripheral benefits from the process.
Not only does burning hazardous waste destroy toxic organic constituents, but it also reduces the sheer volume of hazardous waste. Incinerators actually reduce the solid mass of the original waste by 80-85% and volume by 95-96%, decreasing the load placed on landfills while preventing potentially dangerous materials from leaching into the environment.
Treatment, Storage, and Disposal Facilities (TSDFs)
Hazardous waste facilities that treat, store, and/or dispose of waste are known as Treatment, Storage, and Disposal Facilities (TSDFs). Hazardous waste incinerators are regulated under EPA’s Clean Air Act (CAA) and Resource Conservation Recovery Act (RCRA). These facilities must have a permit to construct and operate.
This permit authorizes the types and quantities of waste a TSDF can accept and the treatment, storage, and/or disposal activities that the facility may conduct. It also outlines operating conditions and recordkeeping procedures the TSDF must follow and regulates the emissions that result from the combustion process (e.g., organics, hydrogen chloride (HCl), particulate matter (PM), and fugitive emissions).
There are currently 22 TSDFs in the U.S. permitted to incinerate hazardous waste.
National Capacity
In December 2019, EPA published its National Capacity Assessment Report, which evaluates the nation’s long-term capacity for hazardous waste recovery, treatment, and landfilling and RCRA-permitted commercial TSDFs. According to this most recent Report, the U.S. has sufficient recovery, treatment, and disposal capacity for managing all hazardous waste generated through 2044.
Despite this analysis, however, consolidation and restructuring in the commercial hazardous waste industry has resulted in fewer RCRA-permitted energy recovery facilities, incinerators, and landfills. Additionally, new federal regulations, permit denials, statutory limits on landfills, changes in fire code requirements, allowed disposal methodologies for certain types of hazardous waste, and changing market conditions all have the potential to disrupt TSDF operations and capacity limits.
The continually changing hazardous waste market is creating a fair amount of uncertainty whether hazardous waste management capacity can actually meet demand. Implications of this are evident in the delays currently being experienced for disposal and incineration. Many Large Quantity Generators (LQGs) and Small Quantity Generators (SQGs) are experiencing a hazardous waste incineration slowdown firsthand right now. Most, if not all, of the permitted TSDF incinerator facilities are currently backlogged several months.
One waste management company KTL works with has received letters from five different incinerators stating they will not approve or accept incineration material for 60-90 days and, most likely, through the end of 2021. There is a backup of hundreds of loads of material to incinerate. Shutdowns and outages for maintenance and rebricking have caused some of these issues. Regulators retracting some storage permits has caused a glut of material in need of immediate processing, as well.
This is causing many fuel-blend/solvent-based incineration-destined waste streams to stack up. This presents great cause for concern for some businesses (i.e., LQGs) that may exceed the 90-day LQG storage limits, as set forth in the CAA. If the backlog worsens, SQGs with a 180-day limit for storing hazardous waste onsite (unless travel to dispose exceeds 200 miles) might also have reason for concern.
What You Can Do
If you are an LQG or SQG being adversely impacted by this backlog and reaching your storage limits, it is important to take the actions necessary to remove the risks of compliance penalties and fines. This starts with:
- Knowing what waste and volumes you have onsite.
- Being proactive. Do not wait to dispose of your waste and allow for plenty of time for scheduling issues. It will be easier to dispose of smaller amounts than larger quantities.
- Evaluating the different disposal alternatives (e.g., fuel blending) and making sure you have secondary disposal options.
- Documenting everything.
If you are in the situation where you are coming up against your time limits, contact your EPA Regional Administrator and ask for guidance on how to manage the situation. Considering writing a letter to the EPA Regional Administrator (ECAD/CB/RCRA) detailing hazardous waste management concerns:
- Include dates, quantities, and waste descriptions.
- Document correspondence with all incinerators you contact.
- Document all other disposal options considered and evaluated.
- Inform EPA of the ongoing plan for safe storage of hazardous waste during the lag in disposal options.
Facilities must keep very careful and accurate records of all hazardous waste information to demonstrate appropriate management. Once the waste is eventually shipped off site, facilities should once again notify the EPA Regional Administrator with details, especially if it takes more than 30 days.
KTL is actively engaged with EPA and having ongoing conversations with hazardous waste disposal vendors to assist our customers through this difficult challenge. The risk of penalty is great, and we are working diligently to provide guidance, support, and regulatory assistance to navigate this situation as safely and compliantly as possible.

FDA’s Focus on Food Traceability
According to the Centers for Disease Control and Prevention (CDC), approximately 48 million Americans—about one in six—get sick with a foodborne illness every year. Of that number, CDC estimates 128,000 end up hospitalized and 3,000 will die from a foodborne disease. Correspondingly, consumers rank safety higher than anything else (e.g., affordability, healthful eating) when selecting a restaurant. Consumers want—and need—to know more about their food.
It is not surprising, given this information, that improving food traceability is a key objective for the U.S. Food & Drug Administration (FDA). Food traceability is the ability to track any food through all stages of the supply chain—production, processing, distribution—to ensure food safety and operational efficiency. Over the past year, the Agency has launched three major initiatives to create safer and more traceable food supply chain.
Read KTL’s recent article in Food Safety Magazine about these initiatives and FDA’s focus on food traceability.

Comments: No Comments
Creating Sustainable Impacts Part 1: SMM vs. IWM
How we use materials and products is a large factor in energy use, climate change, raw material consumption, and our economic stability. Correspondingly, our consumption habits play a major contributing factor to all these statistics, as cited by the U.S. Environmental Protection Agency (EPA):
- Between 1970 and 2004, worldwide greehnouse gas (GHG) emissions increased by 70%.
- The U.S. consumed 57% more materials in the year 2000 than in 1975.
- With less than 5% of the world’s population, the U.S. was responsible for about one-third of the world’s total material consumption from 1970-1995.
- In 1900, 41% of materials used in the U.S. were renewable. By 1995, only 6% of materials consumed were renewable.
- Of all the materials the U.S. consumed in the past 100 years, more than half were consumed in the last 25 years.
As developing nations continue to industrialize and increase their material consumption, resource demands and pressures on our supply chains will only increase. According to EPA, “the implications of current patterns of material use for the environment (including climate), the economy, and our survival are profound and unsustainable.”
But it is possible to stop this pattern from continuing along this path.
The Triple Bottom Line
Most entities are familiar with the triple bottom line (TBL) as a framework to measure performance that goes beyond traditional financial metrics to also measure social and environmental performance. At its core, the TBL is a system where economic growth is tied directly to factors that reduce environmental impacts, encourage social justice, and generate financial returns. It is also one of the best indicators of how sustainable an organization is.
By adopting sustainable materials management (SMM), organizations can improve their TBL—reducing their environmental impacts significantly, while still increasing profit—and contribute to the overall sustainability of our world.
Sustainable Materials Management vs. Integrated Waste Management
Identifying and managing wastes is important. If waste is incorrectly managed, there are regulatory compliance risks, exposure risks, and potential financial penalties that can have lasting impacts. This is what Integrated Waste Management (IWM) is about—managing materials after they have reached the end of their useful life and keeping materials out of the landfill to the extent possible.
SMM broadens the ideas behind IWM to examine all the environmental impacts of material production and consumption, not just waste diversion or recyclability. It considers the entire lifecycle (i.e., extracting, manufacturing, distributing, using, and end-of-life management) of a product and/or process. EPA expands on this concept stating, “SMM is an approach to serving human needs by using/reusing resources productively and sustainably throughout their lifecycles, generally minimizing the amount of materials involved and all associated environmental impacts.” And, subsequently, contributing to the TBL.
EPA cites several ways SMM is different than current IWM approaches:
| Sustainable Materials Management (SMM) | Integrated Waste Management (IWM) |
| Seeks the most productive use of raw materials and resources. | Seeks to minimize and/or manage wastes or pollutants. |
| Focuses broadly on impacts of all the lifecycle stages of a material or product (upstream, midstream, and downstream). | Focuses on what to do with wastes once generated (downstream). |
| Concerned with inputs and outputs from/to the environment. | Concerned mainly with outputs to the environment. |
| Goal of overall long-term system sustainability. | Goal of managing a single set of environmental impacts. |
| Responsible parties include everyone involved in the lifecycle of a material or product, including consumers. | Responsible parties are those who generate waste. |
Regulatory Drivers
The Resource Conservation and Recovery Act (RCRA) provides the legislative basis for EPA’s SMM Program. RCRA establishes a preference for resource conservation over disposal. EPA’s Waste Management Hierarch further emphasizes source reduction/waste prevention and reuse over the options of recycling and composting, energy recovery, and treatment and disposal.
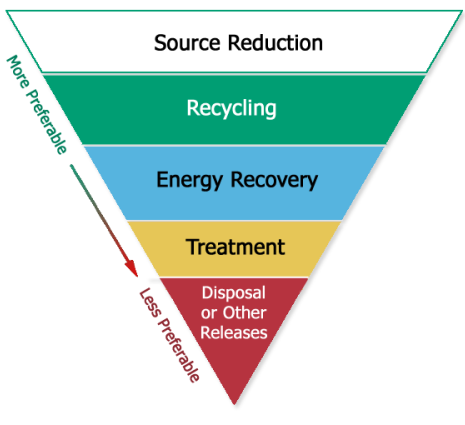
Even with these preferences, the current U.S. environmental regulatory requirements focus largely on controlling end-of-pipe emissions to the air, water, and the land. The regulatory system does not focus on sustainability; as such, current environmental regulations do not require a lifecycle focus when it comes to waste management.
Despite the lack of regulatory requirements, EPA is working to promote efforts to manage materials and products from a lifecycle perspective through the U.S. EPA Sustainable Materials Management Program Strategic Plan: FY 2017-2022 (October 2015) and the related Sustainable Materials Management: The Road Ahead (June 2009) document. The Agency reinforces the need to identify new approaches and better integrate programs to address how materials are extracted and subsequently designed, manufactured, used, and managed at end-of-life to ensure there are sufficient resources to meet not only today’s needs but also those of the future.
One of the best ways an organization can help achieve these goals is to conduct a lifecycle analysis (LCA), which considers potential environmental impacts at every stage of a product’s life. Part 2 of KTL’s series on Creating Sustainable Impacts will dive into conducting the LCA.

Comments: No Comments
Staff Spotlight on Kasia Branny, Esq.
Get to know our KTL team! This month, we are catching up with KTL Senior Consultant Kasia Branny. Kasia’s expertise is in international and U.S. regulatory and legal compliance. She has worked for nearly 15 years as an attorney and EHS regulatory consultant for clients across the globe. Kasia is based out of the Washington, D.C. metro area.
Tell us a little bit about your background—what are your areas of expertise?
I have a law degree from Jagiellonian University in Krakow, Poland and an LL.M. from the George Washington University in Washington, D.C. I joined KTL in January 2021 as a Senior Consultant supporting some of our government contracts, as well as food safety and EHS projects. Prior to joining KTL, I worked with a number of consulting companies as an attorney and regulatory consultant helping clients across the globe address their EHS compliance issues. I have specialized expertise in advising companies on regulatory compliance in the U.S. and internationally; developing regulatory compliance programs; creating audit/assessment content, protocols, and tools; and providing regulatory compliance training.
What types of clients do you work with? What are the biggest issues you see them facing right now?
I work with a diverse cross-section of KTL’s clients—from USAID to food companies needing food safety support to EHS projects for manufacturing organizations.
For several of our clients, one of the biggest struggles they encounter is a limited budget. Unfortunately, this can put new projects on hold until funding is available. With respect to many of the EHS projects I have worked on, COVID-19 has presented many challenges. From regulatory perspective, restrictions/new requirements adopted and imposed on industry have required innovative solutions to comply. As many have experienced, the restriction on onsite audits is one challenge we have had to navigate with our clients. Some audits have been suspended, but in many cases, onsite audits have been replaced by remote audits. We are finding remote audits continue to provide a very viable alternative for many of our clients, even as the COVID restrictions are lifted.
What would you say is a highlight of your job?
The most rewarding part of my job is definitely having a client who is happy with our services and who recommends us to others or for future work.
Another highlight for me is the opportunity to learn new things, which I do on daily basis. Food safety is a new area for me, so there is much to learn! I really enjoy diving into this field, and I have witnessed over the past several months a great interest in the development of electronic Food Safety Management Systems (FSMS). We see many companies in the food industry struggling with data management. For example, just think about the documentation food companies must collect from all their suppliers. That task alone becomes much more efficient with some sort of electronic data management tool. KTL’s Microsoft SharePoint® system provides a simple and intuitive tool that can be easily integrated into a company’s existing operations. There are many data sets and compliance/certification requirements that can be captured, tracked, and managed in a SharePoint FSMS to ensure ongoing compliance.
What do you like to do in your free time?
I enjoy working out and try to exercise at least five times a week. I love nature, so I regularly take my kids and my dog for walks, hikes, and trips to the beach in the summer. With my international background, traveling is my passion. Now that COVID restrictions are being lifted, I’m hoping to start traveling again!
Read Kasia’s full bio.

DOT General Awareness Training: June 16 & 22, 2021
Department of Transportation (DOT) code (49CFR172.702) requires that any employee involved in the transportation (shipping or receiving) of hazardous materials must be trained and tested in general awareness, safety, site-specific job functions, and transportation security.
8-hour DOT General Awareness Training (ONLINE)
June 16 (part A) & June 22 (part B), 2021
8:30 am – 12:30 pm CT
REGISTER NOW!
KTL’s 8-hour DOT General Awareness Training (held as two 4-hour sessions online on June 16 and June 22) is applicable for all companies that ship hazardous materials, ship hazardous waste, or prepare shipments of hazardous materials/waste for transport. It teaches all topics required for DOT general awareness training and general security training and will meet the requirements for triennial training certification.
Topics covered include:
- Code training requirements
- Shipping papers
- Hazardous materials table
- Incident reporting
- Hazard classes
- Common violations and confusing specifics
- Marking, labeling, and placarding
Cost: $198/participant. This online DOT training is held as two 4-hour sessions: June 16 (Part A) and June 22 (Part B) from 9 am – 1 pm. To receive CERTIFICATION, participants MUST complete Part A and Part B and pass both post-tests with 80%.
Training Details
- Training sessions will be held via Zoom. Link will be provided prior to class.
- Training is scheduled to begin at 8:30 am and end at 12:30 pm CT (or until material is complete).
- Participants will receive a training manual, pre-/post-competency test, exercises, and a certificate of completion, provided they receive an 80% or above on the test.
- Registration closes 72 hours prior to the scheduled training. KTL has the authority to cancel training with 72-hours notice if class size is not large enough.

Comments: No Comments
Staff Spotlight on April Greene
Get to know our KTL team! This month, we are catching up with KTL Consultant April Greene. April is an experienced EHS professional with a history of working in the environmental services industry. She has significant experience creating and managing corporate programs, plans, policies, and procedures to ensure compliance with EHS and food safety requirements. April recently moved to our Madison, Wisconsin office.
Tell us a little bit about your background—what are your areas of expertise?
I held various science-related positions working while getting my degrees. I have done everything from making and testing butter to testing sewage and wastewater. I obtained my Master of Science degree in Environmental Chemistry, with a concentration in Toxicology and Hydrology, while working as an Assistant Supervisor of an inorganic environmental laboratory. Most recently before joining KTL, I spent almost five years as an EHS Specialist in the electronics recycling industry. This is an industry that changes frequently with the technology produced, requiring me to stay current on a lot of different types of regulations.
My work background has taken me in and out of the foods industry, which I am still involved in at KTL. My passion, however, remains environmental work and finding sustainable solutions to progress toward a more circular economy, including reuse and recycling. Regardless of the work I’m doing, I enjoy coming up with creative solutions for industry that are outside the box but fit the client’s unique needs. As just one example, one of my favorite projects from my past work involved using glass from recycled electronics as a base for artesian Italian tiles.
What types of clients do you work with? What are the biggest issues you see them facing right now?
I am extremely lucky to not have a “type” of client that I work with. I shift back and forth between organizations of all kinds that have either EHS or food safety needs (or both), which keeps my creative brain fueled. The biggest issues I see them facing right now involve trying to find cost-effective ways to do the right thing. People want to do what is best for the environment and for their communities, but they don’t always know where to start. That is where I come in!
What would you say is a highlight of your job?
My favorite thing is the sigh of relief that comes when my clients realize that I am here to help. I am lucky that I have amazing colleagues at KTL to work with. We work as a team to bounce ideas off each other and to make sure we find the best solution available for our clients.
What do you like to do in your free time?
I have 14-year-old and 4-year-old kids, so free time is not something get a lot of! I am a nerd at heart who loves reading. There is not a genre of book that I will not devour. When I am traveling, I listen to podcasts. When I am with my extended family, we really enjoy playing games together, especially Dungeons and Dragons.
Read April’s full bio.

EHS Compliance: Top Issues
Companies committed to environmental, health, and safety (EHS) compliance face a complicated array of federal, state, and local regulations that may vary by industry sector, facility size, setting, and location. Technical EHS compliance has undergone significant changes over the last several of years—and more is likely to come in the foreseeable future. The evolving EHS landscape presents some significant challenges that companies must address to remain in compliance.
Pandemic
The COVID-19 pandemic has certainly impacted EHS, as it has other operations. There are probably few organizations that have not implemented operational changes on some level to respond to the pandemic—whether that has involved more remote working situations for staff, increased or decreased production, or updated travel and health and safety guidelines.
Changes such as these have had a cascading impact on the way organizations and EHS operations work. With more staff working from various and often remote locations, Cloud-based access EHS and facility documents, records, and shared applications has become essential. Employees need access to everything regardless of location. Along the same line, virtual monitoring methods have also become a necessity. With new guidelines for travel and who may be allowed in a facility, in-person monitoring, assessing, and auditing to meet EHS compliance requirements may not be possible for some facilities.
After over a year of adjusting to a new way of operating through the pandemic, resuming “normal” operations can present additional challenges. Workplace culture has undoubtedly changed. Defining what the culture is as individuals may (or may not) return to the work environmental will requirement management of change and, likely, training. It is important for organizations to address workplace changes and expectations and to evaluate new ways of doing business.
Staffing
EHS department understaffing has long been reported as an issue. In a 2016 study done by Triumvirate, 72% of companies reported EHS understaffing. Many organizations do not have dedicated EHS resources, and many EHS departments often consist of one individual who fulfills multiple roles. Internal resource growth as operations resume is questionable, as EHS expertise can be expensive. This presents an even bigger issue with many experienced workers—often those with the facility EHS background—electing not to return to the workplace full time. This is an area where EHS compliance efficiency and tracking tools are becoming essential to allow companies to do more with fewer resources.
Regulatory Uncertainty
Not surprisingly, EHS regulations—climate change, air, waste, water—are undergoing seismic shifts with the new Administration taking office. Some of these notable changes include the following:
- Environmental Protection Agency’s (EPA’s) new Waters of the U.S. (WOTUS) Rule
- Major Lautenberg Law Amendments to the Toxic Substances Control Act (TSCA)
- Chemical Safety Board’s (CSB’s) new Chemical Release Reporting Rule
- Latest Clean Air Act (CAA) requirements for facilities
On top of this, the differences between state and federal regulations are growing in many states. Organizations need to understand what requirements are applicable and what must be done to maintain compliance at all levels.
Enforcement
From 2017-2020, the U.S. experienced the lowest number of Occupational Safety and Health Administration (OSHA) inspections in over 10 years—including fewer complex investigations. In this same period, the Agency also has had the fewest OSHA inspectors conducting inspections in 40 years.
Not surprisingly, COVID has stalled many enforcement activities and court cases. However, despite COVID, EPA issued approximately $3 million in fines in Q3 of 2020:
- > $1.5 million Resource Conservation and Recovery Act (RCRA)
- > $1 million in Clean Air Act (CAA)
- > $0.5 million in Clean Water Act (CWA)
With the new Administration and resumed business activities, the frequency of comprehensive multimedia environmental inspections appears to be increasing. EHS regulatory enforcement is regaining momentum and likely will continue over the next few years.
Facing the Challenges
Achieving and maintaining EHS compliance requires great management and expertise to ensure all aspects of a company’s technical compliance have been identified and are being actively managed. A management system can provide the organizing framework to enable organizations to achieve and sustain their operational and business objectives through a process of continuous improvement. Information technology (IT) can further help to carry out daily tasks, connect staff, manage operations—and play a vital role in managing compliance requirements.
A compliance information management system brings IT and management systems together to coordinate, organize, control, analyze, and visualize information in such a way that helps organizations remain in compliance and operate efficiently. A system like this will help provide operational flexibility, generate business improvement, and prepare organizations to address these and other EHS compliance challenges that will continue to surface.

Comments: No Comments
Food Safety Magazine Article: Food Remote Audits
Audits provide an essential tool for improving and verifying compliance performance. Audits may be used to capture regulatory compliance status (e.g., FDA, USDA); certification system conformance (e.g., FSSC 22000, SQF, IFS, BRC); and adequacy of internal controls, potential risks, and best practices.
Most regulations, standards, and certification programs require audits to be conducted with some established frequency. For many food companies, figuring out how to meet these audit requirements amongst travel restrictions, new company safety protocol, and government quarantines related to COVID-19 has presented a significant challenge.
The Online Alternative
Fortunately, the Global Food Safety Initiative (GFSI) and the benchmarked certification schemes have responded to this challenge, recognizing that online/remote/virtual audits can offer a viable alternative to onsite audits—even when companies are not operating in a pandemic.
Read KTL’s recent article in Food Safety Magazine about remote food safety auditing and best practices for doing it right.

Comments: No Comments
RMP and Section 114 Requests: Are You Prepared?
As a facility environmental or plant manager, one of the most daunting letters you can receive is a Section 114 request from the U.S. Environmental Protection Agency (EPA). Under Section 114 of the Clean Air Act (CAA), EPA is authorized to require facilities to provide information about their operations. EPA can then use that information to develop new emissions standards or, as the case may be, to determine whether a facility is in violation of a rule or standard.
Section 114 Requests
Under the new administration, EPA sent out a Section 114 request earlier in 2021. This request asks facilities questions pertaining to compliance with Section 112(r) of the CAA, which requires facilities that store or use enough of a hazardous chemical to develop and implement a Risk Management Plan (RMP), as codified in 40 CFR 68.
While EPA normally asks for Section 114 responses within 30 days, they are providing leniency because of the COVID-19 pandemic. Despite the additional time, many facilities receiving this letter may not have the background to understand the requirements of the RMP program, whether their facility is in compliance, and how to respond to EPA’s request.
RMP Program
The RMP program was developed in the 1990s. RMP regulates approximately 12,500 facilities, including agricultural supply distributors, waste/wastewater treatment facilities, chemical manufacturers and distributors, food and beverage manufacturers, chemical warehouses, oil refineries, and other chemical facilities.
The goal of the RMP program is to prevent accidental releases of toxic substances that can cause serious harm to the public. To do this, the program requires subject facilities to develop and implement an RMP for their specific operations. According to EPA, “The RMP rule requires facilities that use extremely hazardous substances to develop a Risk Management Plan which:
- identifies the potential effects of a chemical accident,
- identifies steps the facility is taking to prevent an accident, and
- spells out emergency response procedures should an accident occur.”
Modeling Analysis
The RMP must include an air dispersion modeling analysis that addresses air pollution impacts from both a worst-case release of a toxic substance (e.g., a storage tank that ruptures and releases all its contents) and an alternative/more realistic release of a toxic substance (e.g., a loading hose that gets unhinged). This modeling establishes how far from the facility potential harmful impacts can occur and then identifies public receptors within that area—locations where the public would be at risk should an accident occur. These public receptors include schools, residences, parks, hospitals, etc.
In most cases, the required modeling is known as “dense gas” modeling, because typically the toxic substances covered by this rule behave as dense gas when they hit the atmosphere. For example, ammonia is liquefied under pressure in many refrigeration systems. If that ammonia is suddenly released to the atmosphere, it forms a mixture of vapor and very fine liquid droplets, and those droplets quickly cool the nearby air such that a cold mixture of air and ammonia vapor is formed. This mixture is denser than air and thus needs to be modeled appropriately. The dispersion model most often used for industries, AERMOD, is not the right model in this case.
Next Steps
For facilities who have received a Section 114 request and/or who are impacted by the RMP, it is important to:
- Understand the hazards posed by chemicals at the facility.
- Assess the impacts of a potential release.
- Design and maintain a safe facility to prevent accidental releases.
- Coordinate with local emergency responders.
- Minimize the consequences of accidental releases that do occur.
KTL has experience working with a broad cross-section of industries impacted by RMP, particularly chemical companies. We have created RMP and General Duty Clause audit protocols, conducted audits and investigation/improvement programs following significant release events. In addition, our team provides Tier II and TRI reporting, writes plans for OSHA and Emergency Response, routinely works with Local Emergency Planning Commissions (LEPCs) to coordinate emergency response efforts and exercises to keep communities informed and safe, and has partnered with Blue Sky Modeling to provide the required air dispersion modeling analysis.
About Blue Sky Modeling LLC
Blue Sky Modeling, LLC (BSM) is a KTL partner specializing in air quality modeling. BSM primarily models emissions of air pollutants using traditional air dispersion models (i.e., AERMOD and CALPUFF) in support of air quality permitting efforts. In addition to traditional air dispersion modeling, BSM also performs both accidental release and noise modeling; negotiates modeling strategies with air quality regulators; teaches air dispersion modeling courses; and provides expert testimony on modeling issues. BSM has modeled every type of source imaginable, including, but not limited to, oil and gas, power generation, smelting, cement, and chemical.
