The food system and supply chains continue to change and evolve at an accelerated pace. That introduces new risks and challenges—and heightens some familiar ones. As we move into 2022, the Food and Drug Administration (FDA) continues to strengthen its focus on the four core elements of its New Era of Smarter Food Safety to address several ongoing challenges in food safety. In addition to those identified in FDA’s New Era, KTL’s food safety experts have identified some of the key trends they are tracking in 2022—along with some guidance on how to prepare for the future.
Food Traceability
Food traceability is the ability to track any food through all stages of the supply chain—production, processing, distribution—to ensure food safety and operational efficiency. There is a real need for standardization, stronger linkages throughout the supply chain, improved communication and recordkeeping, and faster response. Notably, one of the core elements of FDA’s New Era is Tech-Enabled Traceability, which involves the use technology to create food traceability advancements. Under this, FDA has taken several actions to reduce foodborne illness:
- The proposed Food Traceability Rule was published on September 23, 2020, as required under the Food Safety Modernization Act (FSMA) Section 204(d), to enhance traceability recordkeeping for certain identified foods beyond a limited “one-up, one-back” traceback approach. The final rule must be submitted by November 7, 2022.
- FDA’s 2021 Foodborne Outbreak Response Improvement Plan sets the stage to “enhance the speed, effectiveness, coordination, and communication of foodborne outbreak investigations” through tech-enabled product traceback, root cause investigations, stronger analysis and dissemination of outbreak data, and operational improvements.
To prepare: Familiarize yourself with FDA’s proposed rule. Review the Food Traceability List (FTL) and start building systems and processes now that address requirements for traceability lot codes, critical tracking events (CTEs), key data elements (KDEs), and recordkeeping.
Food Safety Culture
An organization’s food safety culture is ultimately reflected in the way food safety is managed in the workplace. A strong food safety culture creates an atmosphere where everyone in the organization is aware of and helps to prevent any process and/or operational issues and deviations that may impact the safety and/or quality of their products. Food safety culture is another core element of the FDA’s New Era. It is also a key component of the GFSI Benchmarking Requirements Version 2020 and, subsequently, is being integrated as a requirement into many of the benchmarked food safety certification standards. Best-in-class food safety cultures have robust systems in place to ensure consistent commitment, communication, procedures, training, performance measurement, and trust.
To prepare: The incorporation of food safety should translate to all aspects of the business. Assess current food safety program elements, identify improvements that are internally desirable and required, and implement those updates that will create a strong food safety culture.
Sustainable Food Management
Wasted food makes up the largest percentage—over 20%—of any one material sent to landfills and incinerators each year in the U.S. With more customers and businesses focusing on making sustainable changes, food businesses need to work hard to ensure less food is being wasted. According to the Environmental Protection Agency (EPA), sustainable management of food is “a systematic approach that seeks to reduce wasted food and its associated impacts over the entire lifecycle, starting with the use of natural resources, manufacturing, sales, and consumption, and ending with decisions on recovery of final disposal.” The EPA, U.S. Department of Agriculture (USDA), and FDA joined forces to address the magnitude of wasted food impacts across the U.S. through the U.S. Food Loss and Waste 2030 Champion program. Efforts to promote sustainable food management have also extended to the state level.
To prepare: A thorough food and packaging assessment can help identify appropriate strategies to avoid waste, cut down on disposal costs, reduce over-purchasing and labor costs, reduce water and energy use associated with food production, and reduce GHG emissions. Based on the outcomes of the food waste assessment, there are some common strategies for reducing wasted food and packaging.
Health & Safety and Environmental Focus
During the pandemic, businesses had to find new ways of working to ensure health and safety. Employee protection will remain a top priority, particularly with potential increases in Occupational Safety and Health Administration (OSHA) funding to make improvements in workplace safety protection for American workers, as well as significant increases in OSHA’s maximum penalties. In addition, 2021 brought a significant uptick in EPA multi-media inspections, enforcement actions, and large penalties for violations, particularly related to anhydrous ammonia storage, risk management, and chemical accident prevention planning. Anhydrous ammonia is widely used as refrigerant in food facilities, including meat, poultry, and fish processing facilities; dairy and ice cream plants; wineries and breweries; fruit juice, vegetable juice, and soft drink processing facilities; cold storage warehouses; other food processing facilities; and seafood processing facilities aboard ships.
To prepare: Make sure you have the processes, programs, and systems in place—and documented—to ensure you are always protecting employees’ safety and health and meeting OSH Act requirements. If your facility uses anhydrous ammonia, you must understand the hazards posed by chemicals at the facility and design and maintain a safe facility to prevent accidental releases.
Compliance Efficiency Tools
Managing the complexities of a management system is challenging. That is because every regulatory agency (e.g., FDA, USDA, OSHA, EPA) and voluntary certification (e.g., GFSI-benchmarked standards, gluten-free, organic, ISO) calls for companies to fulfill compliance requirements—many of which overlap. Supply chain and internal requirements can create further complications and confusion. And more frequently than not, companies may not understand or have the resources to manage everything that needs to be in place to satisfy requirements. This is an area where compliance efficiency and tracking tools are becoming essential to allow companies to do more with fewer resources.
To prepare: An integrated compliance management system (CMS) is intended to bring various tools together to create one system that effectively manages compliance requirements, enables staff to carry out daily tasks and manage operations, and supports operational decision making by tracking and trending data that is collected daily by the team charged with implementation.
Food Fraud
In May 2016, FDA issued its final rule on Mitigation Strategies to Protect Food Against Intentional Adulteration (IA). While this is not a new rule, supplier approval/verification programs, vulnerability assessments, and written food defense plans will remain a key focus as a surge in food demand and lack of supply has created an environment ripe for food fraud. It is likely that FDA IA inspections could also ramp up, especially when more onsite inspections resume.
To prepare: Develop a food defense plan. This should include conducting a vulnerability assessment and developing the required programs to remove the risks of IA and food fraud.
Others to Watch…
- Food Irradiation. Food irradiation is a food safety technique used to kill pathogens, including bacteria, viruses, and parasites, and extend shelf life. Irradiation provides excellent safe food processing. FDA continues to research and approve irradiation for a variety of foods. Despite consumer resistance, expect this trend to continue.
- Food Investments. We are seeing more and more private equity firms investing in food companies and, subsequently, in their food safety infrastructure. Food safety is a critical factor in these acquisitions. Expect a thorough assessment of the condition of operations (i.e., personnel, equipment, processes, plant); level of food safety compliance/certification status; and any other potential risks that would impact the transaction.
- Cannabis. The regulatory framework for managing cannabis production is still murky. However, this is a rapidly growing market, and we anticipate progress—whether at the state level or federal level—in the development and implementation of regulations and controls (e.g., Good Manufacturing Practices (GMPs), labeling requirements, etc.).
- Labeling. COVID significantly increased the number of new food preparation and delivery/takeout services and ready-to-eat (RTE) food products. These new business models heighten the importance of appropriate labeling, particularly regarding allergens and instructions for safe food production.
Set Your Goals for 2022
As FDA continues to push its New Era of Food Safety forward and new challenges surface, it is important to understand the current landscape, set priorities, and commit the appropriate resources to ensure long-term sustainability. Consider undertaking the following activities:
- Implement a CMS to help coordinate, organize, control, analyze, and visualize the information necessary to remain in compliance and operate efficiently.
- Conduct third-party assessments to provide an outside perspective of food safety systems and compliance/certification to identify gaps in programs that need development/updates.
- Explore technological advancements that allow for further digitization and promote more timely and accurate collection and management of important data.
- Conduct root cause analysis, as needed, to identify underlying issues and ensure similar problems do not occur in the future.
- Build a strong food safety culture that focuses on changing from a reactionary to a preventive mindset that promotes safety and quality.
If you would like help evaluating your current food safety risk level and assessing your priorities for 2022, please contact KTL.
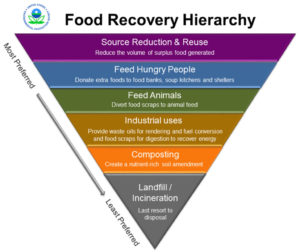 to reduce packaging).
to reduce packaging).