
MECC 2025: KTL to Present on OSHA Fundamentals, IT Tools & EHS Challenges
KTL is excited to be joining the 2025 Midwest Environmental Compliance Conference (MECC) in Overland Park, Kansas, September 15-16, 2025, as a sponsor, presenter, and exhibitor. MECC takes a fresh, regional approach to the increasingly difficult task of environmental compliance, permitting, enforcement, and other critical environmental issues that impact Midwest facilities and institutions.
KTL will be leading the following sessions as part of the workshop’s technical agenda:
OSHA Fundamentals for Advancing Environmental Professionals
September 15, 2025 | 8:45 am | Presenter: April Greene, CSP, CHMM, Senior Consultant
Moving from environmental specialist to EHS leader means you suddenly need to know about fall protection, lockout/tagout, confined spaces, and more. This session gives environmental professionals the strategic safety overview on critical safety programs.
IT Tools for EHS Compliance: Demo
September 15, 2025 | 11:15 am | Presenter: Joseph Kunes, Consultant
Building an EHS IT system doesn’t have to be complicated or expensive. Most companies already
have the software they need. Learn how to build data management tools to collect, track, & report EHS
compliance information using the latest Microsoft 365 and Power Platform apps. See examples and
hear from EHS/IT experts on how you can benefit from this approach.
Navigating EHS Challenges in the Food Industry: Panel
September 16, 2025 | 9:40 am | Moderator: Joe Tell, Principal
In the fast-paced and highly regulated world of food production, EHS professionals play a critical role in maintaining operational excellence while protecting workers, consumers, and the environment. This panel brings together experienced EHS managers from across the food industry to share real-world insights, lessons learned, and practical strategies for overcoming today’s pressing EHS challenges. The session will offer takeaways to strengthen your EHS programs and drive continuous improvement.
And be sure to stop by and visit our booth in the exhibit hall. We look forward to seeing you at MECC!

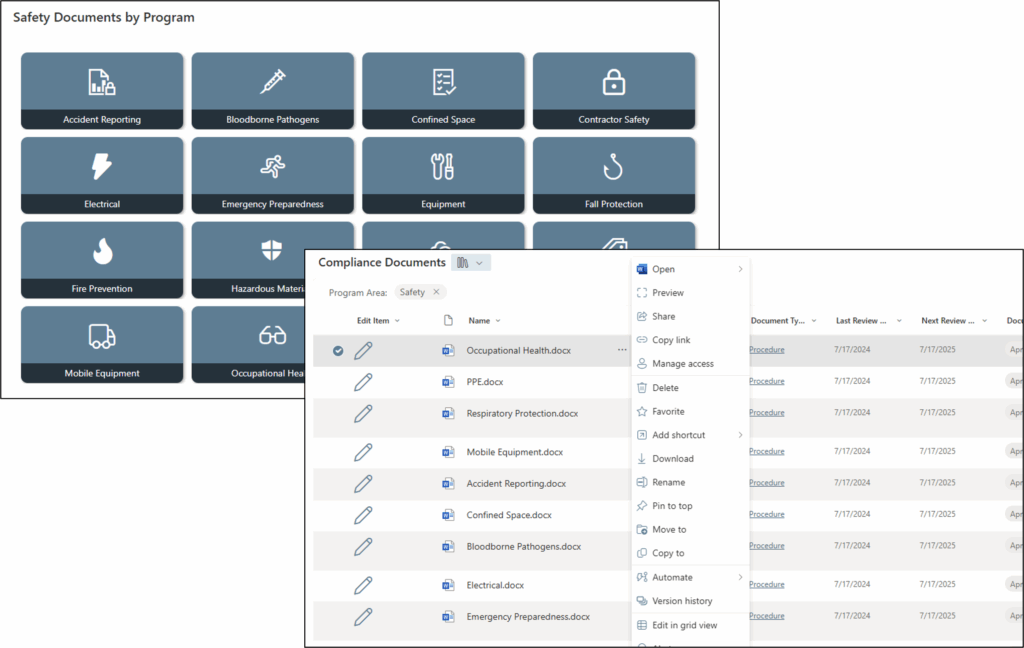
Tech Corner: Safety Document Management System
Functionality: What does it do?
A Safety Document Management System provides a company-wide framework for central, secure storage and organization of safety-related documents and records. This includes policies, procedures, training records, inspection reports, and regulatory documentation to comply with Occupational Safety and Health Administration (OSHA) requirements.
Safety records often have strict retention and security requirements and must be accessible for an audit at any time. A structured Safety Document Management System can help ensure that these critical files are not only retained according to OSHA requirements, but also are standardized, easily searchable, and available to the right people when they need them.
Benefits: Why do you need it?
Implementing a Microsoft SharePoint-based Safety Document Management System provides the following:
- Centralized access to safety policies, procedures, checklists, inspection logs, and training records – whether onsite or remote.
- Document version control to promote consistency and reduce errors associated with outdated versions being used.
- Quick document retrieval, version history, and clear audit trails to demonstrate compliance during audits.
- Improved operational collaboration through real-time share, review, and update capabilities.
- Enhanced workflows configured for notifications, document retention/archive processes, and document approvals (e.g., new procedures, corrective actions) with clear accountability.
- Increased visibility into safety performance by linking documents with incident reports, risk assessments, and corrective and preventive actions (CAPAs).
- Ease in uploading documents (like a shared network drive) and adding/updating metadata to improve searchability.
- Reduced physical paperwork.
- Option to include digital signatures on required documents.
- Better security and access permissions/control to protect sensitive safety data (e.g., incident investigations or medical information).
Together, these capabilities lead to safer workplaces, stronger compliance performance, and reduced risk during audits or inspections.
Technology Used
- SharePoint
- Power Automate

Comments: No Comments
FSSC Development Program
Every company has an obligation to its customers to provide safe, quality, and legal food. Certification under a Global Food Safety Initiative (GFSI)-recognized food safety management program is a growing requirement within key food product supply chains—one that is driven by customer/market demands. The GFSI system provides a high degree of confidence that food safety management systems (FSMS) are adequately designed, implemented, and maintained.
The requirements of the GFSI-benchmarked certification schemes can be complex, and the certification process can be long, especially for small and medium-sized enterprises (SMEs). Initiatives such as the FSSC Development Program can serve as an entry point for organizations to help implement a conforming FSMS without the additional challenge of achieving full certification.
What Is the FSSC Development Program?
The FSSC Development Program provides a flexible approach to food safety conformity, allowing SMEs to demonstrate to the food supply chain and their customers that food safety is a priority. The FSSC Development Program is aligned with Hazard Analysis and Critical Control Points (HACCP) according to CODEX Alimentarius, GFSI Global Markets, and the food safety elements of ISO 22000 and the related sector technical specifications.
The Program offers a series of clear steps to help organizations develop an effective FSMS and provides a pathway to achieve full FSSC 22000 certification, while building the maturity of the FSMS. The Program applies to SMEs in the following sectors:
- Food and feed manufacturing
- Catering and food service
- Retail, wholesale, and e-commerce
- Transport and storage
- Packaging (new as of May 2025 – see below)
How Does It Work?
FSSC outlines the following proposed steps for achieving conformity under the FSSC Development Program:
- Reflect: SMEs review the Program requirements and then complete a self-evaluation using the free Self-Assessment Checklists. Each sector listed above has its own checklist to assess sector-specific requirements. The self-assessment requires SMEs to consider their operations, focusing primarily on existing FSMS elements and prerequisite programs. A second-party auditor may provide additional value conducting this initial assessment.
- Prepare: Once the SME has completed the self-assessment and made appropriate changes, the organization will work with an FSSC Development Program Conformity Assessment Body (CAB) to schedule a complete assessment of the FSMS. The CAB will work with the SME to identify and help to address any nonconformities identified.
- Conform: After a successful assessment, the CAB will issue a Conformity Statement, including any corrective actions needed. This statement serves as evidence of improvements the SME has made toward achieving full certification. At this point, the organization will be listed on FSSC’s List of Conforming Organizations. To maintain conforming status, an annual assessment is required. Once the SME reaches this level, it can continue conforming to the Development Program or proceed toward attaining full FSSC 22000 certification.
What Are the Version 2.0 Updates?
For companies interested or already involved in the FSSC Development Program, Foundation FSSC released a series of improvements (Version 2.0) on May 20, 2025 to better support conformance. Based on the results of a global survey, FSSC updated the Program for the following reasons:
- Include Category I: Packaging in the Program’s scope.
- Adjust the duration of the conformity assessment to better suit SMEs.
- Provide enhanced support to participants, including guidance documents, training, and webinars.
- Increase the Program’s awareness and visibility throughout the marketplace and, correspondingly, strengthen acceptance of the Program throughout the supply chain (i.e., specifying organizations and large buyers).
The primary changes in Version 2.0 include the following:
- Transition to a single conformity level—versus two different levels—aligned with the current Level 2 in Version 1.1.
- Implementation of a full HACCP system aligned with ISO 22000, including critical control points (CCPs) and operational prerequisite programs (OPRPs).
- Shift from a question-based structure to a requirements-based structure in the self-assessment.
Assessments against Version 2.0 went into effect on June 1, 2025; however, conformity assessments against Version 1.1 may still be conducted until May 31, 2026, when everything will transition to Version 2.
Why Participate in the Program?
The FSSC Development Program is ideal for smaller organizations that want to develop and improve their FSMS but might not have the resources to achieve GFSI-recognized certification in one step.
- The Program provides SMEs with a flexible solution that aligns their conformity status to their food safety and business goals (e.g., meeting their clients’ food safety requirements or expanding their market access).
- Integrating the Development Program into vendor assurance programs helps larger food organizations ensure the food safety of their non-GFSI certified SME suppliers through an internationally recognized program.
- Conforming organizations are listed on the FSSC website, making it easy to share conformance status; this global recognition can support expansion into new markets.
- Alignment with the FSSC Development Program supports progression to GFSI certification, if desired, while building the maturity of the FSMS.

Comments: No Comments
From Policy to Practice: Maintaining H&S Compliance
Safe + Sound Week
Maintaining Occupational Safety and Health Administration (OSHA) compliance is not just about avoiding fines—it’s about creating a safe and productive environment where workers can thrive. And it’s about moving beyond paperwork and policy development to implementing practical strategies and creating a culture that makes safety a daily priority.
Practical Strategies
Implementing effective safety protocols and maintaining OSHA compliance requires a combination of diligence, training, regular monitoring, and engagement.
- Diligence. To create an effective Safety Program, you need to understand your regulatory requirements. Equally important, you need a thorough understanding of your operations, your workforce, and your hazards and risks to develop a Safety Program that meets your specific needs. Diligence requires staying informed about changes to OSHA regulations and industry best practices to ensure your facility remains compliant and workers stay protected according to the law. A comprehensive hazard assessment can help identify areas where specific safety improvements—sometimes beyond the law—are needed at your facility. Use this assessment, as well as your understanding of your operations and your employees, to develop or update your Safety Plan and procedures. Procedures should be customized to address specific risks within your facility, while still meeting regulatory compliance requirements.
- Training. A hallmark of any best-in-class organization is its ability to continually look for opportunities to improve. Safety is no exception. OSHA has significant training requirements for employees for good reason. If employees don’t know how to do their job safely, there are real risks of noncompliance, poor employee morale, injury, or worse. Ensure all employees are trained in relevant OSHA standards, understand their responsibilities related to job tasks and safe behaviors, and know how to identify and report potential safety issues. Ongoing education and engagement are key to retention. Safety training shouldn’t be a one-time event; rather, it should be a continuous process that evolves as the facility and industry standards change. Effective training will incorporate interactive learning approaches, real-world scenarios, peer-to-peer learning and mentorships, and continual feedback to create a culture where employees understand and value the importance of safety.
- Regular Monitoring. Regular audits and inspections of your operations, equipment, and practices provide a valuable means of supporting ongoing safety performance and compliance. Every Safety Program needs to be continually reviewed and assessed to ensure it is meeting organizational goals and compliance requirements. Internal audits and inspections can help identify problems so corrective/preventive actions can be put into place and then sustained and improved over time. Many organizations conduct internal audits with their own staff to assess conformance and identify opportunities for improvement. It can be beneficial to bring in a second party at least annually to provide a fresh set of eyes and an objective assessment of overall compliance status.
- Engagement. Engaging workers in safety initiatives, encouraging them to voice concerns, and creating an open dialogue about safety will create buy-in and foster a strong safety culture. Every employee can bring something to the table when it comes to safety. New employees offer a fresh set of eyes; long-term employees have valuable lessons learned to share. Organizations need to continually provide opportunities to get all employees involved in safety in meaningful and appropriate ways, such as creating employee safety committees, involving employees in procedure development, and engaging employees in incident investigations.
Prioritizing Employee Safety
It takes practical strategies to maintain ongoing compliance and create a workplace culture that truly values employee safety. You can make health and safety compliance a daily priority by implementing clear strategies, staying informed on regulations, and actively involving your team. Prioritizing employee safety is about more than just addressing regulatory compliance, it is about safeguarding your employees and the future of your business.

Comments: No Comments
Beyond the Barrier: Machine Guarding
Safe + Sound Week
Machine guarding consistently ranks among the Occupational Safety and Health Administration’s (OSHA) top 10 safety violations. While generally toward the bottom of the top 10, machine hazards are not to be ignored, as they often result in serious injury, amputation, or even death. Machine guarding is crucial for preventing injuries such as these.
Contrary to what many believe, machine guarding is not just a manufacturing issue—it is a fundamental safety control across a wide range of industries, including food processing, warehousing, logistics, packaging, printing, and even some service sectors. Despite the diversity of equipment and processes in these industries, the principles of machine guarding—protecting workers from points of operation, moving parts, and flying debris—apply universally.
Background on Machine Guarding
29 CFR 1910.212 outlines requirements for machinery and machine guarding. According to OSHA, any machine part, function, or process that may cause injury must be safeguarded. Machine guarding refers to those physical barriers, protective devices, awareness barriers, or other safety mechanisms that protect workers from hazards created by moving machine parts.
While some may argue that the Machine Guarding Standard is intentionally vague to apply to the various machine types and configurations used throughout industry, the regulations specify the following requirements:
- Employers must ensure machines are equipped with one or more types of guards (e.g., fixed, interlocked, adjustable, self-adjusting, etc.) that prevent access to hazardous areas while the machine is in operation.
- Machines with moving parts that perform cutting, shearing, punching, or pressing operations must have securely fixed guards that cannot be easily tampered with or removed to prevent employees from contacting the point of operation.
- Workers must be protected from hazards associated with power transmission components (i.e., accidental contact and entanglement with belts, gears, chains, pulleys, etc.).
- Machines must be equipped with emergency stop devices to quickly shut down equipment in an emergency or when a hazard is detected.
- Workers must be trained in safe machine operation, how to recognize hazards, and effective use of machine guards. Regular maintenance and inspection of machine guards are also essential.
Machine guarding is a priority for OSHA, with two National Emphasis Programs (NEPs) aimed specifically at addressing machine hazards and numerous Regional Emphasis Programs (REPs) that are focused in some part on enforcing the Machine Guarding Standard.
Best Practices to Realize Benefits
Machine guards are intended to protect employees. When appropriately implemented, they can not only help reduce injury, but they may also contribute to maintaining regulatory compliance, improving employee productivity, fostering a positive safety culture, and enhancing overall morale.
The following best practice strategies can help organizations in any industry realize these benefits:
- Identify Hazards: A hazard/risk assessment is crucial for identifying all potential hazards associated with each machine. The assessment starts by generating a list of all machines and then identifying any rotating gears, cutting blades, belts, pinch-points, and other dangerous components that could cause harm if not properly guarded. In addition to a general hazard assessment, facilities should also consider regularly conducting the following:
- A Task Hazard Assessment (THA) is a pre-job inspection to evaluate job-specific hazards and ensure appropriate safety precautions, controls, operator training, and other preventive measures are in place prior to job startup. A THA is intended to verify the proper function and design of machine guarding devices before every work shift.
- A Job Safety Analysis (JSA) analyzes potential hazards at each job step that could be introduced by tools, equipment, work practices, or the work environment. JSAs should be performed for all positions before work begins to clearly identify, assess, and implement effective machine design and hazard controls for specific jobs.
- Use Consensus Standards: Consensus standards, such as those developed by the American National Standards Institute (ANSI), provide valuable guidance for understanding and implementing effective machine guarding. These standards are developed by industry experts and stakeholders to reflect current best practices and technological advancements. While not always legally binding, they are often referenced by regulatory bodies like OSHA and can serve as a benchmark for evaluating the adequacy of guarding on specific types of equipment. Using these standards helps facilities identify appropriate guard types, placement, and operational safeguards; ensure workers are protected from hazardous moving parts; and maintain operational efficiency.
- Select, Customize, and Install Machine Guards: Appropriate guards need to be selected and implemented based on the risk assessment. Guards should be suitable for the specific machine and operation and carefully evaluated for ease of use, durability, and level protection. Customization may be required to ensure guards fit the specific operations and provide adequate protection while not compromising efficiency. The effectiveness of the machine guard relies on proper installation. This is not the time to throw away the manufacturer’s guidelines! Adhere closely to instructions to ensure guards are installed and integrated seamlessly with their machinery.
- Develop Proper Procedures: Document the procedures employees must follow when operating machinery. These written procedures should outline how to safely operate equipment, document identified hazards and the associated controls that are in place to protect employees, establish safe practices for equipment maintenance and repair, provide training requirements, etc.
- Train Staff: All workers should be trained in safe machine operation and guard use. Training should cover all points of the written procedure described above, including potential hazards and the essential functions of guard. Importantly, training should emphasize the importance of not bypassing or disabling guards under any circumstances. In addition to understanding safe machine operations, employees should be trained to identify safe and unsafe employee behavior. Many machine guarding-related injuries occur due to unsafe behaviors vs. unsafe equipment, such as bypassing machine guards, performing quick maintenance without following machine guarding procedures, or ignoring procedures to get the job done faster. Employees should be empowered to report this behavior.
- Conduct Inspections, Maintenance, and Monitoring: Once a guard is in place, regular maintenance and inspections will help ensure they remain effective. A Maintenance Program should:
- Establish regular inspection and maintenance schedules.
- Identify and assign qualified inspectors/personnel with appropriate training.
- Develop inspection checklists for each machine/machine guard configuration, integrating requirements from OSHA’s Machine Guarding Standard.
- Create a mechanism to quickly report and respond to findings/nonconformances and assign corrective actions.
- Capture, document, and report machine hazards and unsafe behaviors so others can learn from them.
Prevention Is Key
Don’t wait for an accident to expose a preventable hazard—inspect your machines regularly and ensure guards are properly installed, maintained, and, importantly, never bypassed. Prioritize machine guarding as a core element of your Safety Program to protect your employees, reduce liability, and stay compliant with OSHA regulations.rogram to protect your employees, reduce liability, and stay compliant with OSHA regulations.

Comments: No Comments
The Evolving Emergency: Keeping Your Plan Current
Safe + Sound Week
From natural disasters, like flooding and wildfires, to civil unrest, pandemics, and cybersecurity threats, the need for a robust and adaptable health and safety Emergency Action Plan (EAP) has never been greater. A well-developed EAP not only protects lives, but it can also help minimize damage, promote operational continuity, and reinforce a culture of preparedness.
Core Components of an Effective EAP
In 29 CFR 1910.38, the Occupational Safety and Health Administration (OSHA) requires that facilities develop a written EAP that includes several defined components to address safety and health concerns in emergency situations. At its foundation, an EAP should clearly outline roles and responsibilities, communication procedures, evacuation and shelter-in-place protocols, resource availability, and recovery strategies.
To be truly effective, the EAP must be:
- Risk-Based – Tailored to the specific hazards facing your location, industry, and workforce.
- Accessible – Easily understood and available to all employees, including translations and alternate formats if needed.
- Tested – Regularly drilled and evaluated to identify weaknesses or confusion in execution.
- Integrated – Coordinated with local emergency services, neighboring facilities, and contractors, as well as with other facility emergency plans (e.g., environmental, food safety, security, transportation, etc.).
- Continuously Updated – Adjusted based on lessons learned from incidents, drills, and changing threat landscapes.
Overlooked Elements
Beyond the foundational components that are required in any EAP, there are a number of critical, yet often overlooked, elements that can make the difference between a successful response and a chaotic one:
- Individuals with Special Needs: Plans must account for employees and visitors with disabilities or access and functional needs. This includes evacuation assistance, accessible communication methods (e.g., visual alarms or sign language interpreters), and accessible shelter areas. Failing to provide this support not only increases risk but may also violate regulatory requirements.
- Communication Failures: During a crisis, cell towers can fail, power can go out, and standard communication tools may be rendered useless. Incorporate redundant systems such as two-way radios, satellite phones, or even predetermined physical meeting points to maintain communication under stress.
- Human Behavior and Psychological Impacts: Emergencies can trigger fear, stress, and sometimes panic. An effective plan anticipates psychological responses and includes measures to address those impacts, such as assigning calm leaders, training staff in basic psychological first aid, and having procedures to manage noncompliance or emotional breakdowns during an event.
- Non-Standard Employees: Temporary workers, contractors, and visitors are often left out of training and drills. The EAP should identify how these groups will be informed, guided, and accounted for in the event of an evacuation or shelter-in-place order.
- Blocks and Alternative Routes: Roadways may be blocked by fallen debris, emergency vehicles, or civil disruptions. Pre-identify multiple evacuation routes and practice using them. Update maps regularly and train all personnel on what to do if their primary route is compromised.
- Impact of Weather: An evacuation that works on a sunny day might fail in a snowstorm or hurricane. Plans must consider the timing, temperature, and weather implications on any response. Stock weather-appropriate supplies and plan indoor shelter options to avoid weather impacts to the extent possible.
- Disinformation or Misinformation: False rumors or misinformation can spread quickly, especially on social media. Designate a trusted spokesperson or internal communication channel for official updates. Train employees to verify information through credible sources and not to act on hearsay.
- Overwhelmed Emergency Services: In large-scale disasters, emergency responders may be delayed or unable to assist. Plans should incorporate tools for self-sufficiency—first aid kits, trained staff, emergency food and water supplies, and basic rescue tools—to buy time until help arrives.
Plan, Prepare, Protect
Emergency preparedness is not a one-time task—it’s an ongoing responsibility that must evolve alongside the world we live in. An EAP created five years ago may not address today’s climate threats, social unrest, or technological vulnerabilities.
Don’t wait for a crisis to expose the gaps and overlooked elements in your EAP. Review and update it regularly to address new and evolving hazards. Engage external support to provide a fresh perspective and new approaches. Conduct ongoing risk assessments, scenario planning, and collaborative reviews. These things will help ensure that your emergency strategy remains relevant and effective and your team is prepared, protected, and ready to respond effectively when every second counts.

KTL to Exhibit at the 155th Congress of Correction
KTL will be exhibiting at the American Correctional Association’s 155th Congress of Correction in Denver, CO, August 21-26, 2025. The Congress features a wide array of workshops and sessions focused on cutting-edge topics for correctional facilities. The educational workshops feature the best in the business, passing on critical knowledge and engaging in important discussions, while the expansive exhibit allows attendees to interact with hundreds of companies serving the correctional industry.
Be sure to stop by and visit KTL at Booth #871. We look forward to seeing you at the Congress of Correction!

Comments: No Comments
The Current Truth: Electrical Safety Basics
Safe + Sound Week
Electrical safety issues have been among the top findings in the Occupational Safety and Health Administration (OSHA) compliance audits KTL has conducted over the past year. Many workers are unaware of the potential electrical hazards present in their work environment when completing tasks that involve electricity. As such, they end up working in either unsafe conditions (e.g., poorly maintained/deteriorating equipment) or unsafe work practices (e.g., using conductive tools, not de-energizing prior to work)—or both. The result can be an OSHA finding or worse, including electrocution, electric shocks and burns, injury from exposure to arcing, and fire from faulty electrical equipment or installations.
Electrical Safety Standards
OSHA and National Fire Protection Association (NFPA) electrical safety regulations are complex. OSHA actually has several standards that address electrical safety. The most notable for general industry is 29 CFR 1910 Subpart S, which is broken into sections on design safety standards, safety-related work practices, safety-related maintenance requirements, and safety requirements for special equipment. If you have electrical equipment, at least some of OSHA’s electrical requirements will apply.
NFPA 70E supports the OSHA standard and fills in gaps, particularly related to qualified workers and hazard assessments. NFPA states that an Electrical Safety Program should develop a risk assessment procedure that outlines processes to identify hazards, assess risks, and implement controls before work begins. NFPA 70E is revised every three years.
Common Electrical Hazards
Electrical energy remains one of the most serious health hazards in the workplace, which is why all workers need to understand the dangers of electricity and how to take proper precautions. The following are the most common electrical safety gaps identified in KTL’s safety audits over the past year:
- Misused equipment. If electrical equipment is not used as designed or according to manufacturers’ instructions, safety features are not reliable.
- Damaged or defective electrical equipment is damaged or defective. When electrical panels, power disconnects, or any other electrical equipment in use at the site is not properly maintained, it can fall into disrepair. If electrical equipment is damaged, defective, or missing components, this may cause unintended contact with electrical components and lead to injury.
- Improperly used extension and flexible cords. Cords are subject to damage. Normal wear-and-tear can loosen or expose wires. In addition, employees do not always use the right type of cord for the intended usage and tend to repair and reuse cords that should be retired.
- Lack of ground-fault protection. Daily use of electrical equipment may lead to wear-and-tear (e.g., insulation breaks, short circuits, and exposed wires). Without proper safeguards, deteriorating equipment can create a ground fault that may send electrical current through a worker’s body.
- Missing path to ground. If the power supply to the electrical equipment is not grounded or the path is broken, fault current may travel through a worker’s body.
Creating Your Program
To eliminate these electrical hazards and their associated risks, it is important for any organization with electrical equipment to develop a written Electrical Safety Program, train employees, implement and enforce safe work practices, and regularly verify the Electrical Safety Program’s effectiveness.
Written Electrical Safety Program
A written Electrical Safety Program identifies the possible electrical hazards and outlines the procedures and controls required to effectively eliminate or engineer them out. Development of the Electrical Safety Program should be preceded by an electrical safety hazard assessment that systematically identifies electrical hazards that may cause harm, evaluates how likely and severe the potential harm is, and determines methods to effectively eliminate or control the harm from happening (i.e., hazard controls).
The Electrical Safety Program should consider safety through design, electrical risk management programs, job observations, refresher training, personal protective equipment (PPE) and tools, auditing, and electrical incident investigations.
Employee Training
It is important to understand that electrical training is not a one size fits all. As a part of an effective Electrical Safety Program, organizations should identify the scope of the electrical work—and who is qualified to do it—and then ensure those workers who have not been trained do not do electrical work. OSHA puts workers into two categories: unqualified workers and Qualified Electrical Workers (QEWs).
- Unqualified workers are those employees who are not trained or permitted to work with live electrical components but may face risk of electric shock (above 50 volts). Unqualified workers must receive training on safety-related work practices that pertain to their job tasks and the inherent hazards of electricity (high voltage, arc flash, lack of guarding, etc.).
- According to OSHA, a QEW is one who has received training in and has demonstrated skills and knowledge in the construction and operation of electric equipment and installations and the hazards involved. Only QEWs are permitted to work on or near energized parts.They must have the skills and techniques to distinguish exposed energized parts from other parts of electric equipment and appropriate training on all electrical safety procedures.
Safe Practices and Conditions
Even with the best Electrical Safety Program possible, if an organization continues to implement unsafe conditions or unsafe work practices, electrical safety accidents will continue. The following list provides best practices for working safely with electrical equipment:
General Electrical Safety Principles
- Always assume that overhead lines and all wires are energized, even if they appear to be insulated.
- Never operate electrical equipment while standing in water.
- Do not use electrical equipment that shocks, smokes, smells, is damaged, or has burn marks.
- Train all workers appropriately.
Power Line Safety
- Contact utilities to identify buried power lines, look for power line indicators, and post warning signs.
- Stay at least 10 feet away from overhead power lines (unqualified workers).
- De-energize and ground lines when working near them.
- Use non-conductive wood or fiberglass ladders when working near power lines.
Tools and Equipment Condition
- Use only equipment that is approved to meet OSHA standards, including double-insulated tools and equipment.
- Visually inspect all electrical equipment prior to use and remove any equipment with frayed cords, cracked casings, missing ground prongs, etc. from service.
- Make sure any altered equipment is compliant.
- Continually audit tools and equipment available for use onsite.
Grounding and Bonding
- Ground all power supply systems, electrical circuits, electrical equipment, and exposed metal parts of equipment.
- In wet/damp locations, ensure electrical circuits or receptacles are equipped with a Ground-Fault Circuit Interrupter (GFCI).
- Use GFCIs on all 120-volt, single-phase, 15- and 20-ampere receptacles.
- Follow manufacturers’ recommended testing procedures for GFCI.
- Frequently inspect electrical systems to ensure the path to ground is continuous.
Cord and Plug Safety
- Use factory-assembled cord sets and 3-wire type extension cords marked with designation codes for hard or extra hard usage.
- Use only cords, connection devices, and fittings that are equipped with strain relief.
- Do not modify cords, repair cords with electrical tape, or use them incorrectly.
- Remove cords from receptacles by pulling on the plugs, not the cords.
- Continually inspect cords used onsite.
Lockout/Tagout (LOTO) and De-energization
- When possible, de-energize electrical circuits before doing any type of work according to proper LOTO procedures.
- Use lockout devices to prevent a circuit from becoming energized.
- Use an AC voltage tester to verify that the electrical power is off.
Verify Effectiveness
It is important to analyze the effectiveness of all electrical safety efforts, including electrical safety hazard assessments, the written Electrical Safety Program, and associated work procedures and equipment. Regular audits and inspections can help ensure work conditions and work practices protect employees and create a culture that understands and promotes the importance of electrical safety.
Building Safe Systems
Electrical safety encompasses many different elements and requires specialized qualifications and expertise to safely manage. As such, bringing in a second set of eyes to review and/or help build the right size Electrical Safety Plan for your facility can be helpful (or even necessary depending on in-house electrical capabilities). Relying on experts with not only electrical capabilities but also overall occupational safety understanding can help ensure you implement practical solutions—from compliance audits to customized electrical safety strategies—that integrate with your corporate Safety Program and operations and make your electrical systems as safe as they are efficient.

Comments: No Comments
The Backbone of Safety: Hazard Assessments
Safe + Sound Week
According to the Occupational Safety and Health Administration (OSHA), a hazard is any source of potential damage, harm, or adverse health effects on something or someone. It is a condition, activity, or substance that could lead to injury, illness, or even death if not controlled. For any workplace—regardless of size, industry, location, etc.—it is important to understand and control workplace hazards. This is done by conducting a hazard assessment.
Importance of Hazard Assessments
One of the primary root causes of workplace injuries, illnesses, and incidents is the failure to identify and control potential hazards. A hazard assessment (also known as a risk assessment) is important because it takes a thorough look at the workplace to:
- Systematically identify those things that may cause harm (i.e., hazards), particularly to people.
- Determine how likely and severe the potential harm is (i.e., the associated risk).
- Decide what measures should be in place to effectively eliminate or control the harm from happening (i.e., hazard controls).
- Create a plan to prioritize hazards associated with a specific activity/task/job and implement identified controls.
Steps to Take
Hazard identification and assessment should include the following steps:
- Collect existing information about workplace hazards. Determine the types of hazards that may be present and who might be exposed. This information can come from sources already available in the workplace (e.g., safety data sheets (SDS), operating manuals, OSHA 300/301 logs, job hazard/safety analyses, safety programs, worker input, etc.) or external sources (e.g., trade associations, labor unions, safety consultants, OSHA, NIOSH, CDC, etc.).
- Inspect the workplace to identify safety and health hazards. The objective is to identify potential hazards and address them before incidents occur. Think about how people work and what practices they follow, how equipment is used, what chemicals are onsite, and what the general condition of the facility is. Generally speaking, workers have the best understanding of the work being done, so they should be engaged in inspections.
- Workplace incidents and near misses provide a clear indication of where hazards may exist. Look at past incident records to help identify less obvious hazards.
- Consider employees, contractors, visitors, and members of the public.
- Workstations, processes, operations, equipment, tools—and their related hazards—can all change, so regular inspections should be conducted.
- Consider physical safety hazards, but also identify workers’ exposure to health hazards, including chemical, physical, biological, and ergonomic hazards.
- Emergencies and non-routine situations present different hazards to manage. Identify foreseeable emergency situations and non-routine tasks and then develop procedures for responding appropriately and safely to potential hazards.
- Use checklists to help ensure the major categories of hazards are addressed (e.g., general housekeeping; slips, trips, and falls; electrical; equipment operation and maintenance; fire protection; work organization and process flow; work practices; ergonomics; emergency procedures).
- Assess the associated risk of identified hazards. Think about the types of incidents that could result from exposure to those hazards. Consider the severity of potential outcomes, likelihood that the event will occur, and the number of workers who might be exposed (i.e., the overall risk). How likely it is that someone could be harmed and how serious it could be? Who might be harmed and how?
- Rank your risks. Assigning priority to hazards based on their risk creates a ranking that identifies which hazards are the most serious and should be addressed first. Ranking risks should consider the probability of exposure and the potential severity of an incident. It requires knowledge of workplace activities, urgency of situations, and objective judgement.
- Identify control options. Risk can be reduced/eliminated by controlling or eliminating the hazard or reducing employees’ exposure to the hazard.
- Review external sources and investigate control measures used at other workplaces to identify options.
- Get input from workers who may be able to suggest/evaluate solutions based on their knowledge of the facility, equipment, and work processes.
- Select controls that are the most feasible, effective, and permanent using the Hierarchy of Controls, which emphasizes engineering solutions first, followed by safe work practices, administrative controls, and then personal protective equipment (PPE).
- Avoid selecting controls that may introduce a new hazard; discuss options with workers to ensure their feasibility.
- Develop a Hazard Control Plan. The Plan should describe how the controls will be implemented. What are you already doing to control the risks? What further action is required? Who is responsible for implementing the control? What is the timeline?
- Address the serious hazards first. While interim controls may be necessary, the ultimate goal is to ensure effective long-term control of hazards.
- Determine whether you can get rid of the hazard altogether. If not, identify what can be done to control the risk so harm is unlikely. This might include redesigning the job; replacing materials, machinery, or processes; reorganizing work; implementing new practices to work safely; or providing PPE.
- Select additional controls to protect workers during non-routine operations and emergencies.
- Assign responsibility and deadlines, conduct drills, track progress, and verify the effectiveness of controls once they are installed.
- Implement selected controls in the workplace. Implement measures according to the risk ranking priorities established in the Hazard Control Plan on a “worst-first” basis. Eliminate or control serious hazards as quickly as possible using interim controls, as necessary, while developing longer term solutions. In addition, promptly implement any easy and inexpensive solutions regardless of risk level.
- Follow-up to confirm that controls are effective. Monitor and track progress in implementing controls.
- Conduct regular inspections and preventive maintenance to confirm controls are operating as intended and still effective, particularly when there are changes to the workplace that could introduce new risks (e.g., staff, processes, substances, equipment).
- Record your findings so you can track and verify results.
- Communicate the results. Workers must be aware of every hazard associated with their job and the controls in place to protect them.
Part of the Plan
Hazard assessments are an integral part of a Health and Safety Management Plan. They help organizations to evaluate hazards and then minimize the level of their risk by implementing control measures to prevent injuries and illnesses, meet legal requirements, and create an overall safer and healthier workplace. Employees should be engaged in the process, as they are generally the most familiar with workplace practices. An external party can offer additional perspective, experience, and fresh eyes in answering the following questions:
- What are the potential hazards?
- What can happen and under what circumstances?
- What are the possible consequences and risks of those hazards?
- How risky are the possible hazards and associated consequences?
- How likely are they to occur?
- How severe?
- Who will they impact?
- How can we control or eliminate our hazards to reduce risks?
- Are our efforts successful?
- Have we achieved an adequate level of risk reduction?
- Is further action required?

Comments: No Comments
Observing Safe + Sound Week: August 11-17, 2025
According to the Occupational Safety and Health Administration (OSHA), safe workplaces are sound businesses. Every workplace should have a safety and health program that includes management leadership, worker participation, and a systematic approach to finding and fixing hazards. This is what it means to be Safe + Sound.
Safe + Sound Week
OSHA’s Safe + Sound program is a year-round campaign to encourage every workplace to develop and implement a safety and health program. During one week every August (August 11-17, 2025), OSHA promotes Safe + Sound Week as a nationwide event to recognize the successes of workplace health and safety programs and offer information and ideas on how to keep America’s workers safe. This year’s Safe + Sound Week focuses on emergency preparedness and response.
Participating in Safe + Sound Week can help companies get their safety and health program started, energize an existing program, or recognize company and individual employee safety and health accomplishments. It is an ideal time to reflect on and recognize the efforts you have made to improve workplace safety and health throughout the year and to focus in on areas of improvement going forward.
KTL’s Series on Investing in Safety
Throughout OSHA’s Safe + Sound Week this August, KTL will be featuring a series of safety-related articles and posts on our blog and social media (i.e., Facebook, LinkedIn, X), including:
- Hazard assessments
- Electrical safety
- Emergency response
- Machine guarding
- Strategies for maintaining health and safety compliance.
Watch for these articles! For more information on what your organization can do to participate and promote a strong safety culture, visit the OSHA Safe + Sound Week website.
