
Comments: No Comments
Building Psychological Safety
Safety Focus
Workplace safety and health goes beyond the physical. A strong safety culture focuses on the whole person—and that includes ensuring psychological safety.
What is psychological safety?
Psychological safety is the shared belief that it is okay to take risks, express concerns and ideas, ask questions, speak up, and admit mistakes in the workplace without fear of being punished or humiliated. According to Amy Edmondson, PhD, Professor at the Harvard Business School, psychological safety is “felt permission for candor.” It allows employees the freedom to brainstorm, challenge the status quo, share feedback, take calculated risks, be vulnerable, and work through disagreements together.
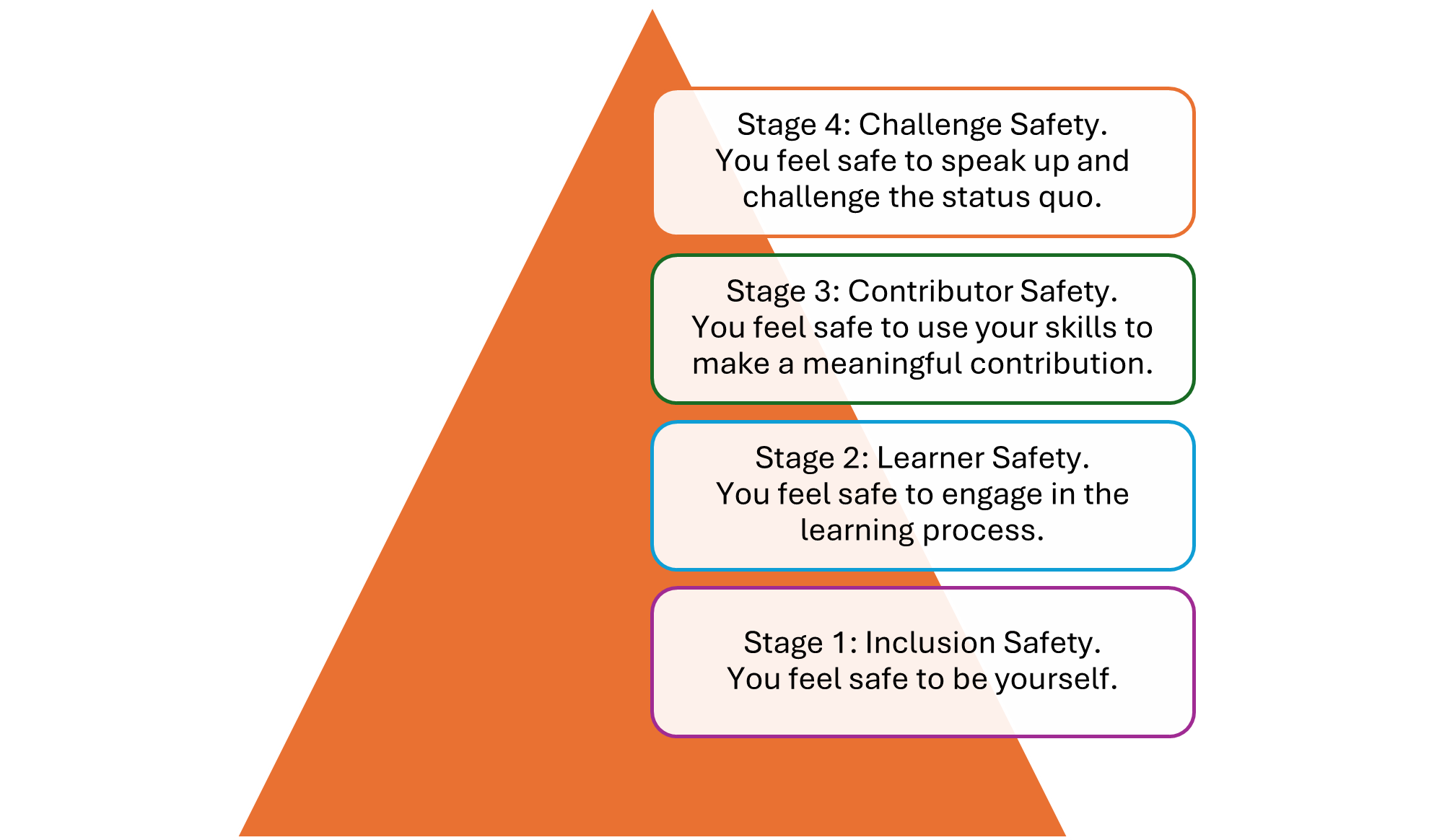
Psychological safety brings in four different dimensions:
- Willingness to help: Employees are comfortable asking for help and trust that their colleagues are willing to provide it.
- Inclusion and diversity: Employees feel included and like their diverse experiences are accepted and valued.
- Attitude to risk and failure: Employees view mistakes as opportunities to learn and grow versus failures to be punished.
- Open conversation: Employees perceive conversations as open and candid; they feel safe speaking up and contributing to discussions.
Psychological safety develops over time. As organizations build greater psychological safety, they move through the following stages:
Why is psychological safety important?
Psychological safety is a critical concept for building effective teams and, many would argue, a critical concept for encouraging workplace innovation and success. According to the Center for Creative Leadership research, teams with high degrees of psychological safety report higher levels of performance and lower levels of interpersonal conflict. This is because employees who feel their workplace is psychologically safe are more willing to engage in behaviors that contribute to greater organizational innovation, including:
- Taking appropriate, thoughtful risks.
- Admitting, discussing, and learning mistakes.
- Openly confronting concerns and tough issues.
- Seeking help and feedback from others.
- Trusting other team members and believing they are there to support them.
- Trusting that they are a valued member of the team whose voice matters.
As psychological safety matures in a workplace, the benefits become more prevalent:
- More engaged team members. When employees can speak up without fear of retribution and feel like their contributions matter, they become more motivated.
- Better and more innovative decision-making. Diverse people voicing their opinions and concerns leads to more diverse perspectives being considered, providing the opportunity for innovation and more informed decision-making.
- A culture of continuous learning and improvement. Teams and individuals grow by sharing mistakes and turning them into opportunities to learn and make changes.
When an organization does not embrace psychological safety, there can be negative impacts on the overall performance of the organization and employee well-being, including stress, burnout, and turnover.
How do I create a psychologically safe workplace?
According to Edmondson, “Leaders must prioritize a culture of learning and innovation for team members to be comfortable speaking up, taking risks, and sharing information…It emerges with effort and curiosity and care.”
A lot goes into creating a psychologically safe environment—and building this culture takes time, focused attention, and good management practices, including the following:
- Make psychological safety a priority. Talk openly with your team about the importance of creating a psychologically safe workplace. Answer questions, dispel misconceptions, explain benefits, and connect it to the higher purpose of the organization innovation.
- Model the behaviors you want to see. Lead by example. Show how to raise concerns and tough issues in a constructive manner. Admit your own mistakes so others feel free to do so; normalize vulnerability.
- Facilitate everyone speaking up. Show genuine curiosity, be open-minded, encourage honesty, and actively listen when someone is willing to challenge the status quo. Make an intentional effort to promote dialogue by asking open-ended questions to get people to feel comfortable speaking up.
- Establish norms for how mistakes are handled. Use mistakes as opportunities for growth; encourage learning from failure by building lessons learned into every project. Do not punish mistakes, experimentation, and reasonable risk-taking.
- Be supportive. Show you are willing to explore out-of-the-box ideas. Embrace productive conflict. Promote constructive debate. Work to resolve conflicts productively. Employ a zero-tolerance policy for any employee deliberately undermining the efforts of another employee.
- Celebrate wins. Acknowledge what is going well, share credit, applaud people’s efforts, and appreciate thoughtful risk-taking. Publicly recognizing and celebrating the unique skills/talents of each team member will build trust and mutual respect.
- Continually reassess. It requires ongoing work and effort to keep your organization psychologically safe. Solicit feedback and track whether you are achieving the results you want.
How do I assess my workplace’s psychological safety?
Edmondson has created a seven-question survey that can be administered anonymously to measure your workplace’s psychological security:
- If you make a mistake on this team, will it be held against you?
- Are the members of this team able to bring up problems and tough issues?
- Do members on this team sometimes reject other members for being different?
- Is it safe to take a risk on this team?
- Is it difficult to ask other members of this team for help?
- Would anyone on the team deliberately act in a way that undermines efforts?
- Working with members of this team, are unique skills and talents valued and utilized?
Psychological safety is not about being nice. Many polite workplaces are not considered psychologically safe, because there is no opportunity for candor and people feel silenced by politeness. It is also not about feeling comfortable all the time. Yes, employees should feel comfortable speaking up, but pointing out mistakes, expressing concerns, and sharing opinions can feel very uncomfortable. Being vulnerable feels risky to most. A psychologically safe culture allows employees to take these risks in a safe environment without negative interpersonal consequences.
More information can be found on psychological safety at the following websites:

Comments: No Comments
Protecting Temporary Workers
Safety Focus
According to the American Staffing Association, America’s staffing companies hire approximately 16 million temporary and contract workers per year. These temporary workers are being more highly utilized than ever before, performing light and heavy industrial labor and other jobs with more complexity and, subsequently, greater health and safety risks. Temporary workers can add value to many businesses; however, using temporary workers should not be considered a cheap or easy fix.
Rights and Responsibilities
Temporary workers are those workers who are supplied to a host employer and paid by a staffing agency. The host employer and staffing agency are joint employers, which means both are responsible for providing and maintaining a safe working environment for temporary workers.
Under the Occupational Safety and Health (OSH) Act, all workers—including temporary employees—have the right to a safe and healthy workplace. To further protect temporary workers, the Occupational Safety and Health Administration (OSHA) launched the Temporary Worker Initiative in 2013, which focuses on ensuring compliance with the OSH Act. The Initiative addresses some common risks associated with joint employment and offers resources in plain language on topics such as health and safety training, recordkeeping, personal protective equipment (PPE), and hazardous communication.
Best Practices
Because temporary workers are jointly employed, the host employer and staffing agency have different health and safety responsibilities, some of which overlap. When hiring temporary workers, effective communication and understanding of responsibilities is vital to ensure workers are adequately protected.
The National Institute for Occupational Safety and Health (NIOSH) and the National Occupational Research Agenda (NORA) Services Sector Council—in partnership the American Society of Safety Professionals (ASSP), the American Staffing Association (ASA), and the Safety and Health Assessment and Research for Prevention (SHARP) program—published Protecting Temporary Workers: Best Practices for Host Employers as a guide to help staffing agencies and host employers better protect the health and safety of temporary workers.
The best practices are organized into the following three sections, each with a checklist to help guide the host employer in its health and safety efforts:
- Evaluation and contracting. Before commencing employment, the staffing agency and host must evaluate all facets of safety and health for the job(s) temporary workers are hired to perform. This includes reviewing all worksites, conducting a joint risk assessment, performing a job hazard analyses, and identifying any necessary training and protection required. Staffing agencies need to be aware of what conditions exist at the worksite, potential hazards that may be present, and how to ensure workers’ protection. This should all be specified in a written contract along with job details, communication responsibilities, injury and illness reporting responsibilities, and other aspects of workplace health and safety. Host employers should also include the staffing agency and temporary employees in any routine supplier or vendor evaluations of performance to minimize liability risks.
- Training for temporary workers and their worksite supervisors. Training is paramount. OSHA requires site- and task-specific safety and health training on approved tasks, hazard identification and control, PPE, OSHA laws/rights, emergency procedures, reporting health and safety concerns/incidents, accessing secure sites, safety and health program participation, etc. Generally speaking, providing this training is a shared responsibility. Staffing agencies conduct general safety and health training that is applicable to different occupational settings; the host provides site-specific training tailored to the hazards and conditions of the workplace. Supervisors should also be trained on approved tasks for temporary workers, process for changing job tasks, mentoring and supervision, OSHA laws, communication and reporting, and joint responsibilities of the host and agency. It is important to make sure supervisors are updated on any changes in temporary worker’s approved tasks and that they understand their responsibility and authority to correct any actions or behaviors that are unsafe.
- Injury and illness reporting, response, and recordkeeping. There should be a procedure for sharing illness and injury information between the joint employers. The host must also set up a method for employees to report work-related injuries and illnesses. If a temporary worker experiences and injury or illness, the host must inform the staffing company of the injury/illness, report it to OSHA, complete required recordkeeping (i.e., OSHA 300 log), conduct a joint investigation with the staffing agency, and coordinate medical treatment/return to work. The host should also have a program to reduce the number and severity of workplace injuries and illnesses and ensure that temporary workers are trained and participate in it.
Research indicates that temporary workers may be more at risk of work-related injury than permanent employees. As temporary workers become a bigger segment of the labor market, temporary worker safety is becoming a larger and more critical issue. Host employers have the legal responsibility to provide all workers with safe and healthy workplaces. This requires recognizing the complex issues associated with employing temporary workers and ensuring they are provided the same rights and protections as permanent employees.
More information can be found on temporary workers at the following websites:

Comments: No Comments
Workers’ Rights
Safety Focus
Every worker in the U.S. has the fundamental right to safe and healthful working conditions. The Occupational Safety and Health Administration (OSHA) created the Occupational Safety and Health (OSH) Act for this specific purpose—to establish and enforce workplace health and safety standards that ensure workplace safety and health protection. Correspondingly, it is employers’ responsibility to provide a safe and healthful workplace that is free from serious recognized hazards, per the General Duty Clause of the OSH Act.
It is important for employees to know their rights and understand what to do if a working condition is unsafe or unhealthy. The following common workplace safety concerns address these rights:
- My workplace is unsafe. If you believe you are working in unsafe conditions (e.g., unsafe machinery, exposure to harmful chemicals, poor air quality, etc.), the first step is to bring those conditions to your employer’s attention. Workers may file a formal complaint with OSHA to request an inspection. Each complaint is evaluated by OSHA to determine whether it should be handled as an offsite investigation or an onsite inspection. Employees have the right for their identities to be kept confidential from employers when filing a complaint. If the environment presents risks of serious harm or death, you have the legal right to refuse to work.
- I don’t have the personal protective equipment (PPE) I need to do my job safely. With few exceptions, OSHA requires employers to provide and pay for PPE when it is necessary to protect employees from job-related injuries, illnesses, and fatalities. This may include hard hats, gloves, goggles, safety glasses, welding helmets and goggles, face shields, chemical protective equipment, and fall protection equipment.
- I don’t know what to do if I get injured at work. You have the right (and obligation) to report any work-related injury or illness. If you are injured, call a supervisor for help. If the supervisor is not available, get medical assistance or call 911. Many companies have procedures for injury response. All employers must notify OSHA within 8 hours of a workplace fatality or within 24 hours of any work-related inpatient hospitalization, amputation, or loss of an eye.
- I don’t know what the hazards are at my workplace. OSHA’s Hazard Communication Standard requires employers to inform and train workers about hazardous chemicals and substances in the workplace. This can be done through warning signs, color-coding, signals, Safety Data Sheets (SDS), and training. It is your right to have this information and to receive relevant training in your native language.
- I want to know my employer’s safety record. Current and former employees have the right to get copies of your medical records, see copies of the workplace injury and illness log, and review records of work-related injuries and illnesses. Employers must give the requester a copy of the relevant record(s) by the end of the next business day. In addition, employers must post a summary of the OSHA Form 300 injury and illness log for the previous year where workers can see it.
- I’m afraid to request a safety inspection or speak with a safety inspector. You have the right to request an OSHA inspection and to talk privately with the OSHA inspector before and after the inspection. A worker representative may also go along on the inspection.
- I was fired or threatened for reporting a safety issue. The OSH Act prohibits employers from retaliating against employees for exercising their rights to file a complaint with OSHA. This is known as whistleblower projection. These rights also cover seeking access to employee exposure and injury records, reporting a work-related injury, requesting an OSHA inspection, speaking to the inspector, and alerting your employer of a safety or health complaint. Whistleblower protection prohibits an employer from taking adverse actions against workers for exercising these rights. A whistleblower complaint must be filed with OSHA within 30 calendar days from when the retaliatory decision was made and communicated to the worker.
- Someone is employing children in an unsafe workplace. Federal child labor law generally prohibits employment of minors in nonagricultural occupations under the age of 14, restricts the hours and types of work that minors can perform under 16, and prohibits the employment of minors under the age of 18 in any hazardous occupation (including the use of unsafe machinery). Different child labor law standards apply to agricultural employment.
OSHA is committed to ensuring safe and healthful working conditions for workers by setting and enforcing standards and by providing training, outreach, education, and assistance. More information can be found on workers’ rights at the following websites:

Comments: No Comments
2024 Safety Observations: Putting Safety First
The Occupational Safety and Health (OSH) Act of 1970 was enacted due to the rising concerns over the high rates of workplace injuries, illness, and fatalities in the United States. Prior to the OSH Act, industrial accidents were common. Reports of hazardous working conditions, coupled with the lack of comprehensive federal safety regulations, highlighted the need for a unified approach to workplace safety. During the 1960s, public and labor organizations further demanded better worker protection. The OSH Act was published to address these issues by establishing consistent safety standards, ensuring safer working environments, and reducing the risk of occupational injuries and deaths.
Since the early days of the OSH Act, occupational safety and health has evolved from being a regulatory obligation to being a core component of how successful companies are run. According to the Occupational Safety and Health Administration (OSHA), having a strong safety and health program can help create:
- Fewer accidents, losses, and disruptions by preventing workplace injuries and illnesses.
- Engaged employees and improved morale.
- Increased productivity and enhanced overall business operations.
- Lower workers’ compensation and insurance claims.
- Improved compliance with OSHA regulations.
- Improved reputation to attract new customers and employees and retain existing ones.
- Better brand and shareholder value that tie to social responsibility.
National Health and Safety Observations
Every year, the National Safety Council (NSC), National Institute for Occupational Safety and Health (NIOSH), and OSHA bring additional attention to occupational safety and health issues to help reinforce the importance of having a strong safety culture and to encourage employers and individuals to recommit to working and living safely.
Every June, NSC and NIOSH observe National Safety Month to encourage employers and individuals alike to be safety role models. This year’s focus areas include safety engagement; roadway safety; risk, reduction; and slips, trips, and falls.
Much like National Safety Month, OSHA’s Safe + Sound Week (August 12-18, 2024) is a nationwide event held each August that recognizes the successes of workplace health and safety programs and offers information and ideas on how to keep America’s workers safe. This year’s Safe + Sound Week is focused on providing resources for businesses to conduct Job Hazard Analyses (JHAs).
KTL’s Series on Investing in Safety
Throughout OSHA’s Safe + Sound Week this August, KTL will be featuring a series of articles and posts on our blog and social media (i.e., Facebook, LinkedIn, X) reinforcing these concepts from OSHA, NSC, and NIOSH and discussing why businesses should invest in safety. Topics will include the following:
- Workers’ rights
- Protecting temporary workers
- Psychological safety at work
- Food chemical safety
- Effective safety leadership
Watch for these articles! For more information on what your organization can do to participate and promote a strong safety culture, visit the websites for OSHA Safe + Sound Week and NSC National Safety Month.

Tech Corner: Property Management Tool
Functionality: What does it do?
As an organization operates more sites/facilities/properties, the need for a reliable tool to manage them becomes more critical. KTL’s Property Management Tool is designed to help users manage and organize property-related documents and information (e.g., contracts, permits, maps, insurance, other legal information) more efficiently. The tool allows users to:
- Maintain detailed information for each property, including property name, address, county, previous owner, date added, insurance carrier, and description.
- Search for properties using a search bar or by selecting alphabetic filters. Once a property is selected, detailed information about the property (as described above) is displayed.
- Associate related documents to each property. This may include contracts, maps, permits, and other legal documents. Each document entry shows the property name, modification date, document type, and document library location.
- Easily navigate between properties, refresh the view, open comment logs, and select properties for further actions.
Benefits: Why do you need it?
KTL’s Property Management App provides the following:
- Enhanced efficiency: Streamlines property management tasks, reducing the time and effort required to access and manage property information.
- Centralized documentation: Keeps all property-related documents in one place, simplifying retrieval and reducing the likelihood of misfiled documents.
- Improved decision-making: Provides immediate access to comprehensive property details, aiding in quicker and more informed decision-making.
- Scalability: Adapts to growing organizational needs and expanding property portfolios.
Technology Used
- Power Apps: Utilizes Microsoft Power Apps to create a flexible and scalable application that integrates seamlessly with other Microsoft services.
- SharePoint: Leverages Microsoft’s robust cloud infrastructure for secure data storage and efficient data management.

Comments: No Comments
Produce Traceability: Uncovering the Gaps in Your Program
The produce industry handles an estimated six billion cases of produce in the U.S. each year. Because a significant portion of this produce travels through the supply chain to reach customers, many produce companies already have some level of traceability program in place. With the finalization of the Food and Drug Administration’s (FDA) Food Traceability Rule, the question is whether these existing traceability programs, systems, and procedures meet regulatory requirements.
KTL’s recent article in Food Safety Tech walks you through how to conduct a gap assessment to determine what requirements your existing programs already meet and identify where improvements are needed to comply with the final Food Traceability Rule by the January 2026 deadline.

Tech Corner: Audit Tool
Functionality: What does it do?
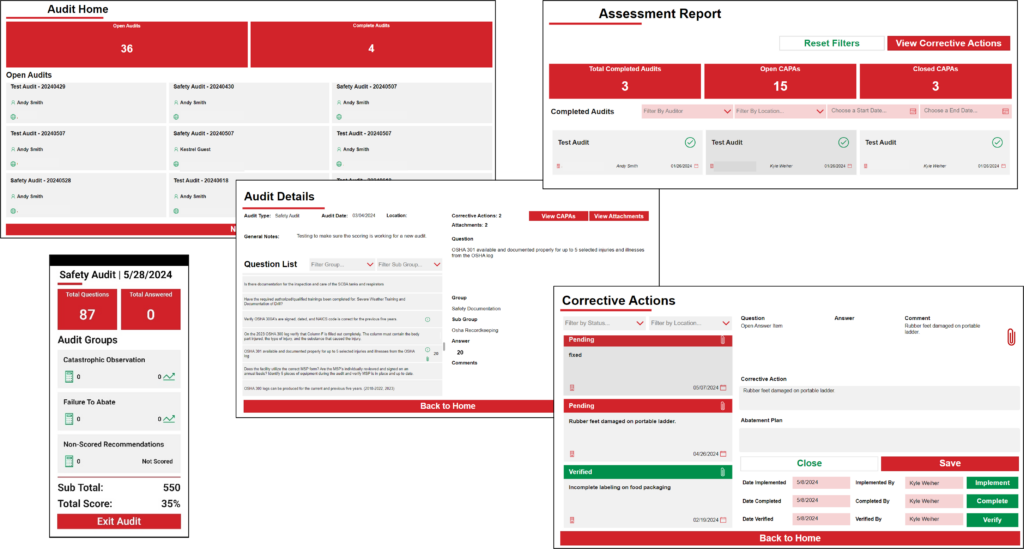
Audits provide an essential tool for improving and verifying compliance performance. Audits may be used to capture regulatory compliance status (e.g., EPA, FDA, USDA, OSHA); certification system conformance (e.g., ISO, GFSI); and adequacy of internal controls, potential risks, and best practices. KTL’s Audit Tool allows organizations to conduct and manage audits. The interface provides a clear overview of completed audits, displays open and closed corrective and preventive actions (CAPAs), and enables detailed filtering to streamline the management process.
Benefits: Why do you need it?
KTL’s Audit Tool allows users to capture regulatory compliance status and certification system conformance. It ensures that audit processes are tailored and responsive by allowing the user to:
- Add new locations and active users; create and modify assessment types; and adjust question templates, scoring values, and answer options.
- Create custom audit templates by selecting the questions to be included in each audit.
- Track audit progress/completion status by subject.
- Quickly gauge the volume of completed assessments and the number of CAPAs that have been successfully resolved and closed.
Technology Used
- Dataverse
- Power Apps

Comments: No Comments
Updates to OSHA’s Hazard Communication Standard
In February 2021, the Occupational Safety and Health Administration (OSHA) proposed to modify the Hazard Communication Standard (HCS) to maintain conformity with the United Nations (UN) Globally Harmonized System of Classification and Labeling Chemicals (GHS) Revision 7, align certain provisions with Canadian and other U.S. agencies, and address issues with the 2012 HCS. Five years later, the final rule to update the HCS was published on May 24, 2024, and will take effect July 19, 2024.
HCS and GHS
The HCS was adopted by OSHA in 1983 to create a standardized approach for communicating about workplace hazards associated with hazardous chemical exposures. The standard requires chemical manufacturers and importers to classify the hazards of chemicals produced and/or imported to the U.S. and to communicate to employees about these chemicals through a Hazard Communication Program, which includes labels and other forms of warning, safety data sheets (SDS), and training. All employers with hazardous chemicals in the workplace must also have a Hazard Communication Program in place to inform employees about these hazards and protective measures.
Like the HCS, the GHS is an international standard that was created as a “universally harmonized approach” to classify chemicals and communicate hazard information. Warning pictograms that can be understood in any language are a key component of the GHS, in addition to universal standards for hazard testing criteria and SDSs. The UN updates the GHS every two years, with the most recent updates in 2023 with Revision 10.
Major Changes
OSHA’s recent HCS updates are the Administration’s first since 2012. The modifications are intended to:
- Improve hazard communication (e.g., precautionary statements) so employees are more aware of the hazards associated with potential chemical exposure and understand how to safely handle, store, and dispose of hazardous chemicals.
- Create a clearer hazard classification process to provide more complete hazard information on SDSs and labels.
- Incorporate new hazard classes and categories.
- Improve clarity and flexibility in requirements (e.g., small packages, packages released for shipment).
The table below highlights some of the updated HCS’s key changes.
| Area | Provision |
| Small package labeling | Provides special labeling alternatives for small containers when it is not feasible to use traditional labels containing full information: * Containers < 100 ml capacity: Use just the product identifier, pictogram, signal word, chemical manufacturer’s name/phone number, and statement that full information is provided on the immediate outer package. * < 3 ml capacity: Only the product identifier needs to be displayed. |
| Trade secret concentration ranges | Allows use of prescribed concentration ranges when exact percentages or ranges are claimed as a trade secret. |
| Health hazards | Updates the skin corrosion/irritation and serious eye damage/eye irritation chapters; adds non-animal testing methods. |
| Physical hazards | Expands physical hazards to include new hazard categories for flammable gases, desensitized explosives, and aerosols. |
| Label elements | Aligns with Revision 7 and includes new or updated hazards, updated guidance, and precautionary statements. |
| SDS | Revises Sections 2 (hazard identification), 3 (composition/information on ingredients), 9 (physical and chemical properties), and 11 (toxicological information). |
| Hazard classification | Clarifies which hazards must be evaluated and the hazard information required on the label vs. the SDS. |
| Packages released for shipment | Allows packages that have been released for shipment to not be relabeled. |
| Bulk shipment | Increases coordination with Department of Transportation (DOT), including allowing labels to be included on immediate containers or with shipping papers, bills of landing (BOLs), or other electronic means that are immediately available to workers on the receiving end of the shipment. |
| New definitions | Provides definitions for the following key terms: bulk shipment, combustible dust, gas, immediate outer package, liquid, physician or other licensed health care professional (PLHCP), released for shipment, solid. |
Compliance Dates
OSHA is taking a phased approach to implementation and compliance with the HCS based on whether the organization handles chemical substances or mixtures. During the transition period between July 19, 2024 (effective date) and the dates below, facilities may either comply with the updated HCS, the 2012 version, or both.
| Date | Requirement | Party | |
| Chemical Substances | July 19, 2026 | Update labels and SDSs | Chemical manufacturers, importers, distributors |
| January 20, 2027 | Update workplace labels, hazard communication program, and training | Employers | |
| Mixtures | July 19, 2027 | Update labels and SDSs | Chemical manufacturers, importers, distributors |
| January 20, 2028 | Update workplace labels, hazard communication program, and training | Employers |
Next Steps
While many of OSHA’s changes will provide additional flexibility to chemical manufacturers and importers, as well as alignment with GHS and international trading partners, the regulatory burden is not insignificant to those impacted. The fundamental structure of the HCS is not changing; however, the revisions will require companies to update SDSs, labels, and training. All of this will take appropriate and resources to meet deadlines. KTL can assist with creating plans and schedules for making these updates to help ensure your facility is ready by the applicable compliance date(s).

Comments: No Comments
Produce Traceability: 4 Steps to Get Started
On November 21, 2022, the Food and Drug Administration (FDA) published the Food Safety Modernization Act (FSMA) Final Rule: Requirements for Additional Traceability Records for Certain Foods (Food Traceability Rule). With the effective date for updated recordkeeping approaching in January 2026, traceability is a top priority for most organizations working in the food industry. Produce companies are especially impacted by traceability requirements as the first step in the food supply chain.
KTL’s recent article in Food Safety Tech outlines four steps to help any produce company prepare to meet FDA’s traceability requirements.

Comments: No Comments
Insights from the 2024 Food Safety Summit
The Food Safety Summit brings together the food safety community to learn more about today’s most crucial elements of food safety—from regulatory concerns and current industry trends to ongoing challenges and the latest technology and solutions.
This year’s Summit, held earlier in May, proved once again to be an engaging and informative meeting for those in attendance. Throughout the Summit, KTL’s food safety experts observed several common themes and challenges that the food industry is facing — challenges that your business may be encountering today. We sat down with KTL’s attendees—Roberto Bellavia, April Greene, Estefania Lopez, and Joe Tell—to get their key insights from the Summit.
What technical topics were covered at the Summit? What seemed to gain the most interest from participants (i.e., “hot topics”)?
The Summit covered a range of content, including food safety culture, artificial intelligence (AI), HACCP, collaborating with regulators, microbial process control and sanitary design, sustainability, root cause analysis, e-commerce, state laws and regulations, viruses and pathogens in foods, food code adoption/harmonization, software, produce safety, and women in food safety. Of these, there were two particularly hot topics we heard about time and again this year: food traceability and cannabis.
Food Traceability. The implementation date for the Food and Drug Administration (FDA) Food Safety Modernization Act (FSMA) Final Rule: Requirements for Additional Traceability Records for Certain Foods (Food Traceability Rule) is January 2026. Impending deadlines have a lot of people talking, as many companies are starting to realize that having multiple software solutions to handle their food safety information is going to make complying with the new traceability requirements a nightmare. Having a robust document/records management system is essential for maintaining the vast number of documents required by regulations and standards, particularly the Food Traceability Rule.
Cannabis. There have been multiple instances of cannabis-infused products with unlisted ingredients falling through the cracks of regulations, creating potential negative impacts on human health and significant reputational issues. This, coupled with the recent information asserting the Drug Enforcement Agency’s (DEA) plans to reschedule marijuana from Schedule I to Schedule III under the Controlled Substances Act (CSA), is pushing many states to protect consumers of food and beverages infused with cannabis in the absence of federal guidance. State regulatory agencies are essentially playing a game of “whack-a-mole” with cannabis companies as they try to navigate where cannabis products fall: Food? Drug? Dietary Supplement? One regulator at the Summit stated that cannabis regulation is a modern day wild, wild west.
Are there any *new* food safety trends you heard about that companies should have on their radar?
There were several talks focused on sustainability at the Summit this year. The FDA, U.S. Department of Agriculture (USDA), and Environmental Protection Agency (EPA) released a joint strategy for reducing food loss and waste for organics in December 2023. They are working to create multiple pathways to make it easier for restaurants, farms, and manufacturers to divert good, nutritious food from landfills to food banks. The strategy also focuses on educating consumers, so they understand how to handle food waste in their own homes.
In addition, there were multiple conversations regarding the significant impacts of global climate change on the food supply chain. ISO’s recent Climate Change Amendments, as well as the Securities Exchange Commission (SEC) Climate-Related Disclosure Rule (which was recently stayed on March 15, 2024) continue to emphasize the importance of accounting for and managing climate change impacts. Companies are at a pivotal point where they need to make decisions regarding their sustainability efforts and how they proceed to meet regulatory and supply chain drivers.
You talked to a lot of different people and companies. What are some of the biggest challenges they are currently facing?
There is a lot of frustration amongst companies, federal regulatory authorities (e.g., FDA, USDA), and state regulatory bodies regarding regulations and guidance for emerging issues like new contaminants (e.g., pathogens, viruses, etc.) and security concerns not being addressed quickly enough. One example brought up during the Town Hall: Real-Time Conversation with FDA, CDC, USDA, and AFDO was the new strain of avian flu being found in raw milk. Because it takes time for testing to be completed—and then subsequent guidance to be created—companies are left wondering what to do about this risk. Most companies cannot very well just stop production during this window of uncertainty without significant impacts. During the presentation, regulators said they are trying to figure out ways to keep up with the speed of change and the associated risks that are constantly present in the food industry.
Lack of support and resources for those managing food safety programs remains an ongoing challenge for many organizations. It is not uncommon for a company’s food safety and quality assurance (FSQA) team/department to comprise only one individual. And while companies seem willing to provide information technology (IT) tools to help manage food safety requirements, many attendees expressed frustration with the lack of harmonization between the various IT solutions, making them ineffective and inefficient to use. See more below.
KTL presented on using existing Microsoft solutions to develop a food safety management system (FSMS). What role do you see IT solutions playing in the food industry?
As stated above, we talked with many companies that have purchased various software solutions with the expectation they would work together; in reality, many of these tools operate independently. As a result, companies are getting frustrated because they have invested time, money, and effort in these tools but have not realized business efficiencies.
There are significant benefits to implementing IT tools that “talk” to each other versus these siloed systems. As our presentation demonstrated, KTL focuses on leveraging Microsoft 365—which most companies are already using—to create integrated systems with various apps/tools that address specific FSQA and operational needs. This approach allows companies to manage various compliance and certification requirements, enables staff to carry out daily tasks and manage operations, and supports operational decision making by tracking and trending data more effectively and efficiently.
Given what you heard, what should companies in the food industry be doing now to plan for the future?
We walked away from the Summit with some key takeaways in some key areas:
Traceability. Companies throughout the supply chain will be impacted by the Food Traceability Rule—potentially even if they don’t have products on the Food Traceability List (FTL). Take time to understand the Rule requirements and how they apply to your operations and to train employees at all levels so they understand their responsibilities. This is an excellent time to perform traceability exercises for everything to help identify gaps, test protocols and verify effectiveness, implement corrective actions, and ensure adequate traceability processes are in place before the January 2026 deadline. It will be especially important to begin or renew communication with contacts throughout the supply chain to facilitate documentation, information sharing, and collaboration. Investing in a good IT solution that integrates with your FSMS will help to further streamline the process.
Cannabis. Companies getting involved in the growing cannabis industry need to stay on top of the rapidly changing regulatory environment. Assess operations, determine what standards might be appropriate, identify gaps in existing programs, prepare for a new regulatory framework—state and/or federal—and begin implementing solutions to eliminate risks.
Food loss and waste. We have all heard reduce, reuse, recycle from the EPA. Now the USDA, FDA, and various food safety certification schemes are holding companies in the food industry accountable for reducing food waste and preventing food loss. Before this becomes mandatory, companies should begin the process of identifying targets and associated methods for reducing food loss and waste.
Food safety outbreaks and recalls. Food safety professionals should be continually monitoring information regarding foodborne illness outbreaks. Companies can reduce their risks by staying informed about emerging food safety threats, identifying and assessing vulnerabilities in their facilities, implementing protocols to help mitigate impacts to consumers, challenging food defense programs by conducting intrusion tests, and developing a comprehensive Crisis Management Plan to manage human health and reputational impacts.
Produce safety. In July 2022, FDA extended the compliance dates for the pre-harvest agricultural water requirements for non-sprout covered produce. Covered farms are subject to the requirements of 21 CFR Part 112 Subpart E if they use water during the growing, harvesting, packing, or holding of covered produce in a way that meets the definition of “agricultural water.” If you fall under this category, note the new compliance dates, which differ depending on farm size:
- January 26, 2025: Very small businesses.
- January 26, 2024: Small businesses.
- January 26, 2023: All other businesses.
Companies receiving produce need to establish the criteria for information that will be requested from the farm upon delivery of produce, such as Certificate of Analysis (CoA). If conducting supplier audits for farms, include the requirements of the Rule into audit criteria.
With all these challenges and trends simultaneously competing for attention—and with fewer resources to manage it all—companies need to assess priorities, needs, and requirements and create a plan for how to meet them.
