Integrated Emergency Response Plans
The most effective way to respond to an emergency is to properly plan for it before it happens. That’s precisely why so many federal, state, and municipal laws and regulations require many facilities to develop and implement some sort of Emergency Response Plan (ERP).
Effective Emergency Response
An ERP is intended to outline the steps an organization needs to take in an emergency—and after—to protect workers’ health and safety, the environment, the surrounding community, and the business itself. The requirements developed by various agencies are important, as they establish the components that must be included in an ERP to comply with regulations and respond effectively.
Most ERPs contain the same basic information. However, it can get complicated when a facility is subject to more than one regulation requiring an ERP, because even though the various regulations share many similarities, they also contain important differences (e.g., command structures, training requirements, equipment needs, operating protocols). Often, facilities end up creating a different ERP to respond to the different regulatory requirements. In an actual emergency, this can create inconsistencies or, worse yet, an implementation nightmare trying to figure out which ERP to follow.
Importance of Integration
The solution lies in integration. For example, consider an integrated management system that allows organizations to align standards, find common management system components (e.g., terminology, policies, objectives, processes, resources), and add measurable and recognizable business value. The same can be done with the various ERPs required within a facility.
It shouldn’t come as a surprise that in 1996, the U.S. National Response Team (NRT) published initial guidance for consolidating multiple ERPs into one core document. The Integrated Contingency Plan (ICP or One Plan) is a single, unified ERP intended to help organizations comply with the various emergency response requirements of the Environmental Protection Agency (EPA), U.S. Coast Guard, Occupational Safety and Health Administration (OSHA), Department of Transportation (DOT), and Department of Interior (DOI). The ICP Guidance does not change any of the existing requirements of the regulations it covers; rather, it provides a format for consolidating, organizing, and presenting the required emergency response information.
While the ICP does not currently incorporate all federal regulations addressing emergency response, it does establish a basic framework for organizations to pull in ERPs for any applicable regulations. And the benefits of doing so are many:
- Streamlined planning process. A single document simplifies the planning, development, and maintenance process. When plans are integrated, it minimizes duplication of effort, eliminates discrepancies and inconsistencies, and helps the organization identify and fill in gaps.
- Improved emergency response. It is much easier—and faster—for emergency responders and employees to navigate one plan rather than multiple separate ones. One plan allows for a single command structure with defined roles rather than potentially conflicting responsibilities. This all allows responders to act quickly and decisively to minimize potential disruption to the organization and public.
- Greater compliance. An integrated ERP provides improved visibility to all parts of the organization’s emergency response and helps reveal gaps that could prove costly and/or dangerous. This is especially important for organizations that must comply with several regulations.
- Potential cost savings. Streamlined and simplified planning reduces the resources required to build the plan. An integrated ERP may also help eliminate regulatory fines and minimize the need for and associated costs of emergency response/cleanup efforts.
Find Your Format
The goal of an integrated ERP is not to create new requirements but to consolidate existing concepts into a single functional plan structure. Regardless of what the plan looks like, it should start with:
- An assessment of the facility’s vulnerabilities to various emergency situations.
- An understanding of the various applicable emergency planning laws and regulations to determine which specific requirements must be incorporated. For example, food emergency response has different implications that must be integrated, particularly when it comes to recovery. A food production facility that is ordered or otherwise required to cease operations during an emergency may not reopen until authorization has been granted by the regulatory authority. Food facilities also have strict guidelines to follow for salvaging, reconditioning, and/or discarding product.
With this understanding, the ERP should then comprise step-by-step guidelines for addressing the most significant emergency situations. The core plan should be straightforward and concise and outline fundamental response procedures. More detailed information can be included in the annex. Most ERPs should include the following basic elements:
- Facility information. Consolidate common elements required in various plans, including site description, statement of purpose, scope, drawings, maps, roster of emergency response personnel, emergency response equipment, key contacts for plan development and maintenance, etc.
- Steps to initiate, conduct, and terminate a response. Outline essential response actions and notification procedures, with references to the annex for more detailed information. Provide concise and specific information that is time-critical in the earliest stages of the response and a framework to guide responders through key steps to deliver an effective response.
- Designated emergency responders. Develop a single command structure for all types of emergencies. Assign qualified, high-level individuals who are familiar with emergency procedures to fill emergency roles. In addition, list the appropriate authorities for specific emergencies, as well as their contact information.
- Evacuation plan/routes and rally points. Clearly mark evacuation routes and identify rally points where employees should meet upon exiting. Do not allow employees to leave designated rally points until it has been documented they have safely left the building.
- Data and information backup technology. Develop provisions for data backup to secondary/off-site systems, as well as alternate options for communications and power.
- Designated plan for communication. Outline who is communicating what, when they are communicating it, and how it is being communicated. This includes internal communication, as well as communication to customers/clients/suppliers/vendors that may be impacted, the media, and the appropriate regulatory authorities.
- Supporting materials. The annex should provide detailed support information based on the procedures outlined in the core plan and required regulatory compliance documentation. Importantly, facilities should create a table that cross-references individual regulatory requirements with the plan to ensure there are no gaps and to demonstrate compliance.
Ensuring Success
The goal of emergency response planning is to minimize impacts to the environment and workers’ health and safety, as well as disruptions to operations. An integrated ERP has the potential to significantly reduce the number of decisions required to respond in an emergency, eliminate confusion and disagreement regarding roles and responsibilities, and enable a timelier, more coordinated response.
That all being said, an integrated ERP will only be effective if it is thoroughly and consistently communicated to all employees. These best practices will help ensure that the integrated ERP functions not only on paper, but also in practice:
- Periodic training is vital to ensure employees understand the ERP and are fully aware of emergency response procedures. It is especially important in an integrated ERP that first responders are trained to handle all potential emergencies rather than more narrowly trained on response for a single regulation.
- Routine drills significantly improve understanding of the ERP, clarify employee roles, test procedures to ensure they work, and diminish confusion during an emergency.
- Posting an abbreviated version of the ERP throughout the plant provides easy access to all employees if an emergency occurs. This summary version of the ERP should highlight the most vital information for quick response: recognized hazards, high-level emergency procedures, evacuation routes, and key contacts.

Comments: No Comments
The Park Doctrine: Holding Individuals Legally Accountable
Since corporations do not act independently, the only way to hold them accountable for a violation and to enforce the law is to hold corporate directors who are responsible for the violations accountable. They may be held to the same standard as the corporation in terms of accountability (United States v. Park, 421 U.S. 658 (1975)).
The Park Doctrine—also known as the Responsible Corporate Officer (RCO) Doctrine—imposes strict, vicarious criminal liability upon RCOs for misdemeanor Food, Drug, and Cosmetic (FD&C) Act violations. The word vicarious is important, because it means RCOs can be penalized without having personally participated in the violation, having been an accessory to it, or even being aware of it. And recently, there have been increasing cases of holding corporate leaders accountable for food safety issues that negatively impact public health.
Setting Precedent
A legal doctrine is a set of rules or principles typically established through legal precedent that courts widely follow. The following two landmark cases set the precedent for the Park Doctrine.
The Park Doctrine has its roots in a 1943 Supreme Court case—United States v. Dotterweich. In this case, the jury determined that Dotterweich, as President of the company, was guilty of FD&C Act violations because he shared in the responsibilities related to the transaction of mislabeled product, even though he had no knowledge of the violation. With this decision, the Court held that individuals could be held criminally liable for the company’s FD&C Act violations.
1975 marks the Supreme Court decision on the United States v. Park. In this case, Park (the President and CEO of a national retail food chain) knew about a rat infestation issue but delegated responsibility to employees to resolve the issue. When it was not resolved by the FDA’s inspection, Park and the company were both charged with FD&C Act violations for introducing adulterated food into interstate commerce. Again, the Court maintained that RCOs are held to strict accountability standards, and—very importantly—liability does not require proof of negligence, intent, involvement, or even awareness of wrongdoing. Rather, liability is based solely on the RCO’s role and responsibilities. Park had a duty to remedy the violations and to implement measures to prevent them—he did neither.
Park Doctrine Criteria
In January 2011, the Food and Drug Administration (FDA) published criteria for recommending Park Doctrine prosecutions that factor in the individual’s position in the company, his/her relationship to the violation, and whether the RCO had the authority to correct or prevent the violation. Specific factors under consideration also include the following:
- Whether the violation involves actual or potential harm to the public.
- Whether the violation is obvious.
- Whether the violation reflects a pattern of illegal behavior and/or failure to heed prior warnings.
- Whether the violation is widespread.
- Whether the violation is serious.
- The quality of the legal and factual support for the proposed prosecution.
- Whether the proposed prosecution is a prudent use of Agency resources.
Holding Individuals Accountable
A broad theme in law enforcement these days is holding individuals accountable, said Mary El Riordan, Senior Counsel in the Administrative & Civil Remedies Branch of the U.S. Department of Health and Human Services Office of Inspector General (HHS OIG). Million-dollar settlements do not change behavior, but holding individuals accountable does.
On September 15, 2022, the Department of Justice (DOJ) released the Monaco Memo, affirming its focus on holding individuals accountable. Deputy Attorney General Lisa O. Monaco stated in her address that the DOJ’s top priority for corporate criminal enforcement is going after individuals who commit and profit from corporate crime. The Monaco Memo is fundamentally about individual accountability and corporate responsibility and represents a desire on the part of the DOJ and FDA to use the Park Doctrine to increase the numbers of criminal convictions of RCOs.
Proactively Mitigating Risks
It is clear the DOJ and FDA are taking an increasingly aggressive stance and prosecuting more RCOs, even without knowledge or intent to commit a violation. In the case of the Price Doctrine, it is a crime to do nothing. It is ultimately the RCO’s duty to be aware of, prevent, and address potential violations.
To do so, there are several best practices companies can take to proactively mitigate risk:
- Implement and maintain an integrated management system (i.e., quality, food safety) to help identify compliance obligations and manage risks through an organized set of policies, procedures, practices, and resources.
- Conduct internal audits. Audits provide an essential tool for continually improving and verifying compliance performance. Routine audits should ensure policies and procedures are being followed and identify concerns before they become major violations.
- Engage in regular monitoring activities. Regular environmental monitoring that focuses heavily Zones 1 and 2 (i.e., food contact surfaces and surfaces directly adjacent to food contact surfaces, respectively) is vital to preventing foodborne illnesses.
- Establish clear roles and responsibilities for all employees, including leadership. Provide training for RCOs on the Park Doctrine so they clearly understand their duties and obligations.
- Resolve corrective and preventive actions (CAPAs) immediately. CAPAs are those actions an organization takes to make improvements and/or eliminate causes of non-conformities. Failure to conduct a CAPA may be considered a major non-conformance.
- Create a culture that encourages employees to speak freely when there are issues. Food safety culture is being integrated more completely and significantly into many of the Global Food Safety Initiative (GFSI)-benchmarked food safety certification standards to encourage widespread adoption.
- Be responsive and work with the FDA. The FDA sends warning letters to companies in violation of the FD&C Act (see example 2022 Warning Letter). The letter specifically outlines the required response and related consequences: You are responsible for investigating and determining the causes of the violations identified above and for preventing their recurrence or the occurrence of other violations. It is your responsibility to ensure your facility complies with all requirements of federal law, including FDA regulations. You should take prompt action to correct or implement corrections to the violations cited in this letter. Failure to do so may result in legal action without further notice, including, without limitation, seizure, injunction, or administrative action for suspension of food facility registration if criteria and conditions warrant.

Tech Corner: CAPA Tracking Log
Functionality: What does it do?
Corrective and preventive actions (CAPAs) are those actions an organization takes to make improvements and/or eliminate causes of non-conformities. The CAPA Tracking Log records, assigns, and tracks CAPAs to closure. This is important, as failure to implement and close a CAPA may be considered a major non-conformance.
KTL’s CAPA Tracking Log also tracks recommendations to improve. Unlike CAPAs, recommendations do not correct a non-conformance; rather, they are suggestions to refine or improve processes.
Benefits: Why do you need it?
The CAPA Tracking Log provides the following:
- Records, assigns, and tracks CAPAs to closure.
- Assigns tasks to specific team members.
- Communicates due dates and sends notifications when tasks are overdue.
- Facilitates verification of completed activities and associated documentation.
- Prevents the occurrence and/or recurrence of issues.
- Optimizes processes and ensures that deliverables are free from defects and other non-conformities.
Technology Used
- SharePoint
- Power Automate

Comments: No Comments
Earth Day 2023: Invest in Our Planet
Every year on April 22, Earth Day celebrates the birth of the modern environmental movement. The very first Earth Day—spearheaded by Senator Gaylord Nelson in 1970—led to the creation of the U.S. Environmental Protection Agency (EPA) and the passage of new environmental laws, including the National Environmental Education Act, the Occupational Safety and Health Act, and the Clean Air Act.
While the early Earth Days concentrated on enacting legislation to clean up environmental concerns, Earth Day today is largely focused on what we can do to sustain our planet for future generations…
Creating a Green Future
The EARTHDAY.ORG 2023 theme picks up from last year—”Invest in Our Planet”—and is focused on engaging governments, institutions, businesses, and citizens to do their part: everyone accounted for, everyone accountable. According to Kathleen Rogers, President of EARTHDAY.ORG, “Businesses, governments, and civil society are equally responsible for taking action against the climate crisis and lighting the spark to accelerate change towards a green, prosperous, and equitable future.”
Government Initiatives
Governments around the globe have enacted many significant green policy initiatives, yet nearly every country in the world is not on track to meet greenhouse gas (GHG) neutrality by 2050, according to EARTHDAY.ORG.
The EPA released its most recent EPA Perspective: 5 Ways EPA is Protecting People and the Planet in honor of Earth Day. The Agency cites the following key areas the U.S. government is concentrating on to positively impact our planet:
- Tackling the climate crisis with the urgency it demands, including technology standards for cars and trucks and funding for local governments to cut climate pollution.
- Confronting longstanding environmental injustices and inequities, through investments in EPA’s environmental justice (EJ) initiatives to fund and support programs to reduce pollution and improve public health, particularly in historically underserved communities.
- Improving air quality in neighborhoods across the country with several action to cut harmful air pollution and smog from power plants and industrial facilities.
- Ensuring clean water for all families through President Biden’s Investing in America agenda, which invests billions in rebuilding our nation’s water infrastructure, and by proposing the first-ever legal limits for PFAS in drinking water.
- Building a healthier future, from the Clean School Bus Program to accelerate a zero-emission transportation future, to starting new cleanup projects at 22 Superfund sites.
What You Can Do
Governments, businesses, and citizens are all essential in taking action to overcome the climate crisis. When it comes to your organization, EARTHDAY.ORG suggests, “Businesses, inventors, and financial markets must drive value for their institutions and society through green innovation and practices. Like other economic revolutions, the private sector has the power to drive the most significant change, with both the necessary scale and speed.”
Change starts with action. Learn more about how you can be part of Earth Day every day.

Tech Corner: Document Management
Functionality: What does it do?
A document/records management system creates a company-wide framework for central and secure storage and organization of and access to documents and records. Having a good document management system is essential for maintaining the vast number of documents required by regulations and standards, particularly since some have retention cycles for as long as 30 years.
Benefits: Why do you need it?
Document management provides the following:
- Central and secure storage, organization, and access to documents and records locally or remotely.
- Process and document standardization.
- Higher quality data due to reduced human error.
- Improved collaboration and dissemination of document updates to staff.
- Improved document searchability and accessibility.
- Enhanced workflows for approving and completing tasks involving documents.
- Easy access for audits and clear audit trail.
- Version control and history.
- Reduced paperwork.
- Ease in uploading documents (similar to a shared network drive) and adding/updating metadata for searchability.
- Improved security of sensitive documents (permissions that can be applied at the document level).
All of which lead to more consistent, efficient, and reliable compliance performance.
Technology Used
SharePoint

Comments: No Comments
Transitioning to FSSC 22000 Version 6.0
Food Safety System Certification (FSSC) 22000 is a complete Global Food Safety Initiative (GFSI)-benchmarked certification scheme that is aligned with the ISO management system approach and harmonized structure. The first version of FSSC 22000 was published in 2009, and more than 16,000 sites have been certified under the scheme since.
On March 31, 2023, the Foundation FSSC published the most recent version of the FSSC 22000 scheme (FSSC V6.0) to:
- Integrate the requirements of ISO 22003-1:2022.
- Strengthen the requirements to support organizations in their contributions to meeting the United Nations’ Sustainable Development Goals.
- Incorporate feedback from the FSSC 22000 V6.0 development survey.
Overview of Changes
Foundation FSSC has set a 12-month period—between April 1, 2023 and March 31, 2024— for companies to transition from V5.1 to V6.0. In addition to changes in some of the requirements (as outlined below), V6.0 includes a realignment of the Food Chain Categories in accordance with ISO 22003-1:2022 and GFSI requirements (i.e., including Trading and Brokering (FII) and removing Farming (A)). Among others, some of the significant changes to the requirements include the following:
Food safety and quality culture. As we are seeing with other GFSI certification schemes, ISO management systems, and the FDA New Era of Smarter Food Safety, culture requirements are becoming more prominent. FSSC 22000 V6.0 requires senior management to “establish, implement, and maintain a food safety and quality culture objective as part of the management system,” addressing the following elements at a minimum: communication, training, employee feedback and engagement, and performance measurement of defined activities.
In addition, organizations must develop and implement a a documented food safety and quality culture plan that outlines objectives and timelines and follows the management system process of continuous improvement (i.e., plan-do-check-act (PDCA)).
Quality control. Quality control is a new clause of FSSC 22000 V6.0 that aligns with clauses 5.2 and 6.2 of ISO 22000:2018. Under this clause, organizations must establish, implement, and maintain a quality policy, objectives, and parameters in line with finished product specifications for all products within the scope of certification. As part of the quality program, organizations also need to establish and implement quality control and line startup and changeover procedures to ensure products meet customer and legal requirements.
Food loss and waste. The regulatory community has identified the lack of circularity in the food industry and a real need for addressing food waste. FSSC V6.0 now requires organizations to develop a documented policy with objectives and detailed strategy to reduce food loss and waste within the organization and the related supply chain.
Equipment management. Organizations must establish and implement a risk-based change management process for new equipment and/or any changes to existing equipment. This includes having documented purchase specifications to address such things as hygienic design, legal and customer requirements, and intended use of the equipment.
Allergen management. The requirement to have a documented allergen management plan is not new to V6.0. However, in addition to a including a risk assessment of all potential sources of allergen cross-contamination and related control measures, V6.0 now requires validation and verification of control measures; precautionary or warning labels as an outcome of the risk assessment to identify allergen cross-contamination risks (warning labels do not exempt the organization from implementing allergen control measures/verification testing); allergen awareness and control measures training; and annual review of the allergen management plan.
Environmental monitoring. V6.0 expands on the previous requirements for organizations to have a risk-based environmental monitoring program (EMP) for relevant pathogens, spoilage, and indicator organisms and documented procedures for evaluating the effectiveness of all controls in preventing contamination. V6.0 requires that the EMP must be reviewed for continued effectiveness at least annually, including when specific triggers occur (e.g., significant changes related to products, processes, legislation; when no positive test results have been obtained over an extended period; and when there is a repeat detection of pathogens during routine environmental monitoring).
Validation/verification of packaging claims. When a claim is made on the product label or packaging, the organization must maintain evidence of validation to support the claim and must have verification systems in place to ensure product integrity.
Food defense. V6.0 clarifies and strengthens requirements related to food defense. Organizations now must conduct and document a food defense threat assessment based on defined methodology to identify and evaluate potential threats linked to processes and products. In addition, the food defense plan must be based on the threat assessment, specify mitigation measures and verification procedures, and be implemented and supported by the food safety management system (FSMS).
Food fraud mitigation. Again, V6.0 clarifies and strengthens food fraud mitigation requirements by requiring organizations to conduct and document the food fraud vulnerability assessment to identify potential vulnerabilities and develop and implement appropriate mitigation measures. The food fraud mitigation plan must be based on the vulnerability assessment, specify mitigation measures and verification procedures, and be implemented and supported by the FSMS.
Other requirements. V6.0 also includes clarifications on the requirements for the certification process and implements the addition of a QR Code on FSSC 22000 certificates for improved traceability. It updates Communication Requirements (2.5.17) so organizations must 1) inform the certification body within three days of serious events/situations that may impact the FSMS and/or the integrity of the certification, and then 2) implement corrective measures as part of the emergency response and preparedness process. In addition, the standard requires that major nonconformities must be closed by the certification body within 28 calendar days from the last day of the audit. If this is not possible, the Corrective Action Plan must include temporary measures and controls necessary to mitigate the risk until permanent corrective action can be implemented.
Next Steps
Sites currently certified to FSSC 22000 have a transition period of 12 months to prepare their FSMS to be audited against the V6.0 requirements. The next year affords these organizations the time to assess current FSSC 22000 elements; identify improvements that are internally desirable and/or required by the new V6.0; and implement those updates to reduce nonconformances with FSSC 22000 V6.0. This can be done through a series of phases to ensure adoption throughout the organization:
Phase 1: Internal Assessment. Review existing FSSC 22000 food programs, processes, and procedures; document management systems; and employee training tools and programs to identify those areas in need of updates, development, and/or implementation to meet the requirements of V6.0.
Phase 2: FSMS Updates. Based on the assessment, develop a plan for updating your FSSC 22000 FSMS, including major activities, key milestones, and expected outcomes. This may include updating/developing programs, processes, procedures, and training with missing V6.0 requirements.
Phase 3: Training. To ensure staff are prepared to implement and sustain the updated FSSC 22000 V6.0 program, they must be trained on applicable requirements; specific plans, procedures, and good manufacturing practices (GMPs) developed to achieve compliance; and the certification roadmap to prepare for future assessments.

Comments: No Comments
Energy Management: Reviewing Your Utility Bill
Energy monitoring is a key first step in effective energy management, as it allows organizations to understand their energy consumption patterns and identify routine waste in the process. Reviewing utility bills is a basic initial action that can offer significant insights into how an organization consumes energy and what opportunities may exist for immediate and longer-term savings.
Uncovering Opportunities
Working with a Certified Energy Manager (CEM) to review and analyze a 12-month history of utility data for both electricity and natural gas can help identify peak demand, load profile, taxes, and historic trends in usage. This information can be used, in turn, to calculate savings on efficiency measures and projects based on utility costs. Such opportunities may include the following:
Tariff Review
A utility tariff governs how an energy provider (electric or natural gas) charges the customer for their electricity and natural gas usage. Utilities set out the rates, terms, and conditions of service in these tariffs. Electricity tariffs can vary widely by location and provider, hence the importance of reviewing the organization’s current tariff with its energy provider.
After reviewing utility bills and preparing a profile of energy usage, it is possible to compare the current tariff with other available tariffs to determine if there is a more cost-effective way to purchase energy. The utility may conduct these tariff reviews on your behalf; however, a second opinion can often identify savings opportunities to take advantage of the way a tariff may be structured. For example, a portion of the total utility bill is typically determined by actual demand and structure of the tariff. Where demand charges are nearing 50% of the bill, there are often opportunities for cost savings, including:
- Shifting production to take advantage of lower rates in off-peak hours or seasons.
- Staggering startups to reduce instantaneous demand.
Tax Exemption
If you are paying the full taxes on your electricity and natural gas, your organization may be eligible for a tax exemption depending on where the facility is located. The tax exemption typically applies to energy (gas or electric) that is used to manufacture saleable goods. Tax exemptions often apply to 75% to 99% of the facility’s total utility tax burden.
To receive the exemption, an energy tax study is required to determine how much energy at the facility is used in the manufacture of saleable goods (i.e., any processes that modify the product) versus the energy used for ancillary operation (e.g., warehousing, welfare spaces, lighting). Typically, these studies must be renewed when significant changes occur at the facility to impact load or every three years, whichever is soonest (depending on state-specific rules). The value of the tax emption can vary greatly depending on facility size.
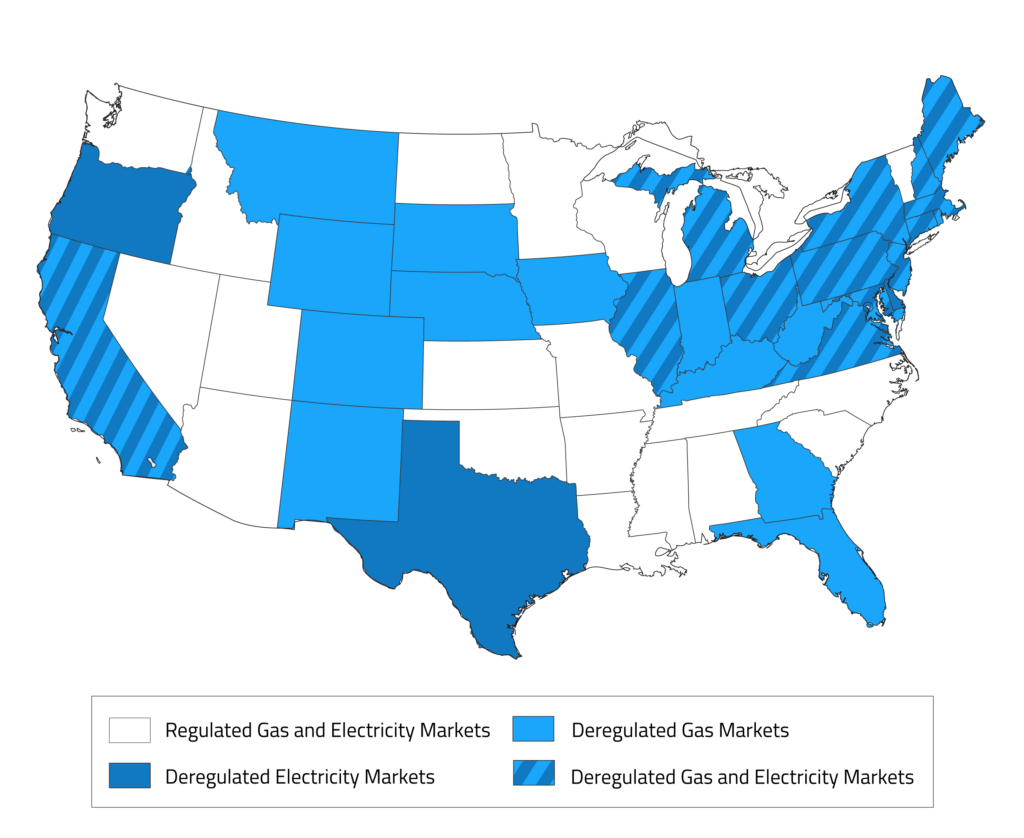
Energy Costs in the Open Market
Energy costs (gas and electric) can be compared against known market rates to determine whether there may be an opportunity for cheaper supply on the open market. The map below shows which states have a deregulated energy market. Any operations in these states may be able to find partnerships with third-party energy suppliers for cost savings.
Utility bills are just a starting point. The more data that can be reviewed and analyzed—and the more detailed it is—the easier it is to identify cost- and energy-saving opportunities within operations.
KTL has experience reviewing utility bills and helping organizations of all sizes identify energy consumption patterns and energy efficiency solutions. Please contact us for assistance in meeting your energy, environmental, and sustainability objectives.

Tech Corner: Dashboards
Functionality: What does it do?
A dashboard is a tool used to quickly display and visualize data, typically using charts, tables, and other graphic elements. Dashboards provide a quick and simplified way for users to understand complex data sets, review key performance indicators (KPIs), and identify trends or patterns.
The data collected in most business processes—ranging from the outputs of an inspection form to the inputs into a companywide sales program—can be displayed in a dashboard. For example:
- Forklift Pre-Op Inspection Dashboard: A dashboard could be set up to track and visually display when the inspection form is being filled out, by whom, what types of issues are being identified, how frequently they are identified, and what outstanding issues need to be closed. These dashboards can help identify trends and improve overall management of the inspection program.
- Marketing and Sales Dashboard: Marketing and Sales departments rely heavily on metrics. Dashboards allow the end user to visualize data that is being tracked, such as advertising return on investment, engagement, growth, revenue, website traffic, sales pipeline, etc. These dashboards can help with sales forecasting and informing marketing decisions.
- Homepage Dashboard: Homepage dashboards are often used as internal landing pages that display important data related to the company and/or specific projects. These might be formatted to contain key metrics, important documents, sales data, assigned tasks, etc. They provide a quick, at-a-glance view of relevant data and information.
Benefits: Why do you need it?
- Dashboards provide an integrated view of data pulled from multiple sources into an easy-to-read display.
- Simplified data visualization allows for improved and more efficient decision-making.
- Dashboards metrics provide for real-time monitoring of data as it is being collected.
- The collaboration, customization, and transparency provided through dashboards help support organizational objectives.
Technology Used
- Excel
- Power Apps
- Power BI
- SharePoint

Visit KTL at the 2023 Food Safety Summit
As one of the premier events in the food industry, the Food Safety Summit provides a comprehensive conference and expo for attendees to learn from subject matter experts, exchange ideas, and find solutions to current industry challenges.
- When: May 8-11, 2023
- Where: Donald Stephens Convention Center, Rosemont, Illinois
- Who: Retailers, food processors, distributors, food manufacturers, growers, foodservice, testing laboratories, importing/exporting, law firms, and other food safety professionals
- Find KTL: Stop by our booth (#534) in the exhibit hall!
KTL Solutions Stage Presentation
Be sure to also update your agenda to attend KTL’s Solutions Stage presentation on Thursday, May 11 at 2:00 pm CT:
The Big Secret: You already have the software you need to build your FSMS.
Having a simple, centralized FSMS to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. It sounds expensive, but it doesn’t have to be—not when most companies already have the software and just need the right combination of food safety and IT expertise to customize it. KTL will present several examples that demonstrate how various food companies are leveraging Microsoft 365® with SharePoint and the Power Platform to elevate their FSMS and effectively manage food safety compliance documentation, data, and certification requirements.

Comments: No Comments
Q&A: Environmental Monitoring for Food Safety
When preparing for compliance and certification audits, environmental monitoring is an area where there are often questions: What are my requirements? How much do I need to do? What happens if I have a deviation? There are many intricacies associated with environmental monitoring that can depend largely on operational processes and compliance and certification requirements—many of which are not always clearly defined. KTL’s food safety experts offer their high-level perspective on some common questions regarding environmental monitoring.
What is environmental monitoring?
Environmental monitoring involves sampling and testing your facility’s environment for pathogens, spoilage and indicator organisms, and allergens to prevent foodborne illness. Environmental monitoring is typically done by swabbing various surfaces for pathogens and sending those samples to an accredited lab for analysis. This monitoring helps to 1) assess how effective the plant’s cleaning and sanitation programs are, and 2) determine whether any pathogens are living in the facility so it can respond accordingly (e.g., adjusting cleaning procedures, addressing personnel hygiene issues, etc.).
What is an Environmental Monitoring Program (EMP)?
An EMP refers to an entire program for organizing the monitoring process to prevent pathogens—and foodborne illness—in finished product. The EMP helps to identify those areas where harmful microorganisms could be harboring in the facility and to implement and verify the effectiveness of pathogen controls (e.g., cleaning and sanitation procedures, sampling frequency and methodology, employee hygiene practices). Ultimately, the purpose of an effective EMP is to help a facility identify and implement strategies to eliminate pathogens and prevent their recurrence.
Who needs an EMP?
Certain foods are considered high risks for harboring pathogens and growing bacteria. These include beef, poultry, dairy, seafood/shellfish, ready-to-eat (RTE) food, baby food, leafy greens, and tree nuts. According to U.S. Public Health Service, the organisms in the table below—and their sources—are the biggest culprits of foodborne illness:
| Organism | Sources |
| Campylobacter | Raw and undercooked poultry and other meat, raw milk, and untreated water |
| Clostridium botulinum | Improperly prepared home-canned foods |
| E. coli 0157:H7 | Beef, produce, raw milk, and unpasteurized juices and ciders |
| Listeria monocytogenes | Unpasteurized dairy products, sliced deli meats, smoked fish, hot dogs, pate’, and deli-prepared salads |
| Norovirus | Any food contaminated by someone who is infected with this virus |
| Salmonella | Raw and undercooked eggs, undercooked poultry and meat, fresh fruits and vegetables, and unpasteurized dairy products |
| Staphylococcus aureus | Cooked foods high in protein that are held too long at room temperature |
| Shigella | Salads, unclean water, and any food handled by someone who is infected with the bacterium |
| Toxoplasma gondii | Raw or undercooked pork |
| Vibrio vulnificus | Raw or undercooked seafood, particularly shellfish |
The kill step is the point in food manufacturing when dangerous pathogens are removed from the product. This is often done by killing pathogens through processes such as cooking, pasteurization, irradiation, and freezing. It is one of the most important steps in keeping food safe. The following questions can help determine whether an EMP may be necessary for your facility:
- Does your process have a kill step that removes dangerous pathogens from the product?
- Is your product exposed to the environment after the kill step and before packaging?
- Does your product combine RTE products without including a kill step?
Why do I need an EMP?
From a compliance perspective, the Food and Drug Administration’s (FDA) Food Safety Modernization Act (FSMA) Final Rule for Preventive Controls for Human Food requires it: “A facility who has identified a potential environmental pathogen or indicator organism as a hazard to RTE foods is required to include an EMP in its Food Safety Plan. A trained Preventive Controls Qualified Individual (PCQI) needs to review EMP test results to ensure that the Food Safety Plan is being followed.”
From a certification perspective, most Global Food Safety Initiative (GFSI) food safety certification schemes also require an EMP, and failure to have an effective program will result in a major non-conformance.
From a consumer protection perspective, environmental monitoring is intended to protect consumers by keeping harmful bacteria from contaminating the food we eat. An EMP can help serve as an early warning system for identifying a potential contaminant before it spreads throughout the facility and into food that reaches the consumer.
What does an EMP include?
EMP requirements are set forth by FDA and GFSI certification programs, but retailers and consumers may also have their own requirements that impact the food supply chain. According to FDA, a FSMA-compliant EMP should include:
- Established, written, and scientifically valid procedures.
- Identified testing microorganisms, adequate locations, and number of collection sites.
- Identified timing and frequencies for collecting and testing samples.
- Identified corrective action procedures in compliance with CFR 21 section 117.150.
- Testing performed by an accredited laboratory.
An EMP should be tailored to the facility’s specific operations and food products; however, there are several steps that every program should include:
- Perform a risk assessment. Determine the risks associated with the plant’s operations, including identifying high-risk foods and potential pathogens that could be present. The frequency of environmental monitoring will be determined by the hazards and risks identified.
- Determine hygienic zones. An EMP should include sampling to assess activities, pathogens, associated risks, and mitigation options within the following zones (from highest to lowest risk):
- Zone 1: Direct food contact services (e.g., counters, conveyers, utensils).
- Zone 2: Indirect food contact surfaces that are close to food contact surfaces (e.g., crevices of equipment, drip shields).
- Zone 3: Indirect food contact surfaces that are not close to food contact surfaces (e.g., walls, floors, drains).
- Zone 4: Areas distant from food contact surfaces and processing areas (e.g., locker rooms, lunchrooms, offices).
- Implement and manage testing protocols. Testing and sampling protocols should identify the frequency of sampling required (depending on risks), number of samples (depending on the facility size), timing of sampling (before/during/after production), person responsible for conducting sampling, and an accredited lab (ISO 17025) to use for testing, as needed. Some facilities may opt to conduct internal “rapid tests” at interim phases of production by trained staff to provide more immediate and cost-effective results and leave third-party lab testing for the final product. Regardless of whether testing is done internally or by a lab, an effective EMP will swab different sites each time to reduce the likelihood of contamination going undetected.
- Develop corrective action procedures. Sampling is intended to identify high-risk areas. How an establishment responds to any findings—and how quickly—is critical. Potential corrective actions may include changing cleaning chemicals, increasing frequency of cleaning program, requiring uniforms, etc.
- Verify and validate the EMP. Data, programs, and procedures should be regularly reviewed to ensure the EMP is serving its intended purpose. Verification provides proof the EMP is working; validation provides proof it is effective.
What do I do if I have a deviation?
The FDA anticipates that facilities with EMPs will occasionally detect environmental pathogens. If this happens, the facility should immediately enact the corrective actions outlined in the facility’s EMP (see above). This may include modifying cleaning and sanitation procedures, recleaning areas, conducting retesting, holding product, or even issuing a product recall.
It is important for facilities to remember that any environment has the potential to become contaminated with a pathogen. Never having a positive result for a common environmental pathogen might not necessarily mean that the EMP is “perfect;” rather, it might be a sign that the right areas are not being swabbed adequately. Keep in mind that any positive result offers an opportunity to improve the EMP and related cleaning/sanitation/hygiene procedures.
How can I prevent environmental pathogens?
There are a number of key actions food processors can undertake to prevent environmental pathogens from contaminating their facilities and food products:
- Apply Good Manufacturing Practices (GMPs). GMPs apply to EMPs, as with every other aspect of a strong food safety program. GMPs that will impact environmental monitoring results include employee hygiene practices, sanitary facility and equipment design, and cleaning and sanitation processes.
- Evaluate, implement, and verify preventive controls. Identify your greatest risks for environmental pathogens, and proactively develop strategies to implement controls in your process flow. These may include controlling pedestrian walkways to avoid personnel contamination; using dedicated tools, equipment, and/or staff post-process; having special uniforms for staff, etc. Just as important, you must verify the performance of your preventive controls through environmental monitoring and take corrective action immediately if problems arise.
- Ensure employees and other resources are qualified. Employees responsible for sanitation, sampling, and overseeing the EMP must have the necessary training and/or experience for assigned duties. In addition, any outside labs used for environmental testing must be accredited under ISO 17025.
- Review and update the EMP. Products, operations, equipment, employees, processes, and other environmental factors change—and all of these can impact the EMP. Conducting periodic reviews to ensure processes and procedures reflect any new conditions is important in ensuring a facility’s overall hygiene and its products’ quality and safety.