
KTL to Present on EHS at REMS Summit 2026
If you are in the Rockford area, you won’t want to miss KTL at the REMS Summit 2026, held at the NIU Rockford Campus on February 5, 2026. The REMS Summit offers Rockford-area leaders in manufacturing keynote sessions on legislative updates and strategic priorities, as well as interactive breakout sessions covering workforce development, automation/AI, supply chain strategy, HR modernization, Lean leadership, and environmental, health & safety compliance.
KTL’s April Greene, CSP, CHMM, will be presenting a breakout session on Environmental, Health & Safety: What You Need to Know. This session will:
- Provide an overview of applicable federal, state, and local occupational safety and environmental regulatory requirements.
- Explain what is required to comply with federal, state, and local environmental and occupational safety regulations (i.e., your obligations as an employer).
- Identify common compliance pitfalls and gaps in existing operations, processes, and practices.
- Outline best practices for developing practical systems to manage risk and stay compliant.


Trends in Technology for 2026
Every year, we see new enhancements, integrations, and tools for effectively integrating technology into business practices. When used appropriately, technology can be used to simplify compliance, create efficiencies, improve recordkeeping, inform better decision-making, and deliver meaningful savings in time, effort, and cost. KTL uses Microsoft 365 (MS 365) and the Power Platform as a dynamic information management suite of tools that can be customized to provide streamlined, centralized information management, and consolidated reporting to all levels of the organization. Here are some of the top Microsoft 365 technology trends and advances KTL anticipates for 2026.
SharePoint Modernization
As KTL has previously reported, Microsoft has been slowly phasing out the classic SharePoint experience in favor the modern experience introduced in 2016. Microsoft is making these changes to the modern experience to provide enhanced functionality, better user experience, and improved site performance. In 2026, this includes removing SharePoint 2013 Workflow from existing tenants (April 2026) and fully retiring and ending support for both InfoPath (i.e., how you create forms) and SharePoint Designer (i.e., legacy SharePoint management and Workflows) (July 2026).
Transitioning from a classic SharePoint site to a modern one is not considered a “simple” process, because there is no one-to-one mapping between the functionality offered by classic web parts and what is offered on modern pages. However, not only is it worth doing, but those who don’t modernize will be slowly left with an unusable system.
Tip: Assess your existing site to determine priorities for modernization and then develop a plan to transition system elements. Consider opportunities for integrations and enhancements available through the modern experience. Leverage external resources to improve functionality, enhance user experience, and create better overall performance.
Paperless Checklists and Forms via Power Apps
Many organizations have a lot of great data at their fingertips, but being able to efficiently access and use it can be a challenge, especially if data is still being collected via paper forms. Mobile forms and digital checklists allow employees to quickly and easily collect data from a mobile device—from daily inspections (e.g., forklifts) to near-miss information—as it is happening in the plant/field. Data is submitted directly to the system and turned into valuable information that can then be further analyzed. Notifications for identified issues and program compliance can also be configured to create greater visibility, and dashboards can help identify concerns and trends to improve compliance and productivity.
With the retirement of old InfoPath forms (see above), Power Apps provides an upgraded forms experience. Power Apps forms are responsive and integrate with MS 365 and modern SharePoint. For example, Power Apps work seamlessly with Power Automate to support automated workflows based on form data.
Tip: Assess where you are still using paper forms and checklists to conduct routine inspections and gather data. Leverage Power Apps to build digital forms to better collect, manage, and use data.
Streamlined Wokflows with Power Automate
Power Automate provides the opportunity to take the routine processes and repetitive steps employees must complete to get the job done and streamline them, maximizing company time and resources, increasing operational efficiency, saving money, and improving overall business productivity.
Workflow automation reduces opportunities for human error and the potential mistakes, inaccuracies, and costs that can accompany it. It creates more efficiency by automatically making assignments and moving projects/documents/tasks along in the process. Being able to easily track a project or process through an automated system can subsequently improve productivity and ensure tasks are completed as planned. A clearly designed workflow helps employees understand their roles in the process and enables cross-functional collaboration. Real-time reporting and performance metrics can further be used to inform decisions and modify processes to improve performance.
Tip: Identify processes and routine tasks that can be automated. Design step-by-step workflows that can be automated in Power Automate to improve business efficiency.
New Premium Tools
Modern PowerApps Controls. A subset of Power Apps modern controls is now available, offering improved usability and templatization. Recent Canvas App control updates make apps more responsive, consistent across devices, and easier to maintain/update. Improvements to layouts, templating, and copy/paste of Power FX logic help speed up development. In addition, these modern controls integrate more cleanly with Microsoft themes.
Dataverse/Premium Capabilities. Most organizations do well with SharePoint and standard licenses. For larger inspection programs, incident tracking, or organizations with many locations, Dataverse and premium features offer advantages, including relational data, stronger security roles/groups, workflows between environments, higher quality reporting, and PDF generation.
Premium Connectors. Power Platform premium connectors extend the capabilities of the Power Platform by enabling secure, scalable connectivity to enterprise systems (e.g., SQL Server, SAP, Salesforce, Oracle, and other line‑of‑business applications). These premium features come at an additional cost but allow organizations to automate more complex processes and work with more data sources. The list of premium connectors—and thus the reach of the Power Platform—continues to expand every year, extending its capabilities and giving organizations additional ways to leverage the Power Platform.
Planner Premium. Many organizations have been using Microsoft Planner to assist their teams in task management and basic project management. Planner Premium moves beyond basic task tracking to true project execution, including timelines, Gannt charts, task dependencies, workload insights, milestones, and agile tools. Planner Premium supports more structured and complex projects, enabling organizations to improve visibility, balance workloads, track progress effectively, and manage work with greater control and accountability.
Tip: Work with an IT expert to evaluate where these advances can help improve your systems.
Artificial Intelligence (AI) Integration
No discussion of technology trends would be complete without addressing AI. Over the past year, Microsoft has continued to expand its AI strategy with the introduction of MS Copilot, bringing AI directly into business workflows. Embedded across MS 365, Power Platform, and other Microsoft solutions, Copilot enables users to work smarter by automating routine tasks, gaining insights faster, and supporting better decision making.
In addition to productivity gains, Copilot and other AI tools are increasingly being used to enhance and transform business processes. AI can add complexity to workflows, enhance Power Apps, classify and route documents, apply optical character recognition, and more. Additionally, AI-driven analysis can be used to review historical workflow and operational data to identify trends and potential opportunities for improvement.
Tip: Identify areas where automation and data analysis can be done by AI. Keep in mind that over-automation without expert oversight can significantly increase error and legal exposure. Use caution exploring where you can use AI for more basic tasks that can be automated, as not all processes lend themselves to AI.
Looking Ahead
Integrating technology into business processes can help organizations stretch and empower every limited resource. The key is identifying those traditional processes that will benefit from technological integration and then using the right technology to create greater efficiencies and enhance business value. With technology advancing a rapid pace, now is the time to:
- Assess your current systems and processes. Where are there opportunities to automate? Where are systems outdated/no longer working? Where can you improve overall business efficiencies?
- Determine how you can integrate technology into your current operational practices to create continuous improvement and competitive advantage. Consider your organization’s immediate issues within the context of overall organizational objectives.
- Leverage partnerships with consulting partners with deep IT and compliance knowledge and practical implementation experience to help strategize; formulate an approach that leverages the appropriate technology, including new advances; and then design and build scalable systems and tools.

Comments: No Comments
2026 Food Safety Trends to Watch
Less federal oversight is a consistent theme across the U.S. government in 2026, and the Food and Drug Administration (FDA) is no exception. Significant budget cuts are impacting federal focus—and corporate requirements, expectations, and obligations—when it comes to food safety, from inspections to rulemaking efforts. Even so, there is heightened pressure globally and from the public to elevate food safety efforts. Here are some of the things KTL’s food safety experts are watching in 2026.
FDA Budget Cuts
As with most federal agencies, the FDA is not immune to reduced budgets for FY2026 under the Department of Health and Human Services (HHS). Cuts include significant reductions in staff, causing FDA leadership to shift focus to streamlining operations and relying on artificial intelligence (AI) tools to fill in gaps. It is anticipated that states may take on more responsibility for conducting routine food inspections due to the lack of federal capacity. In addition, federal budget cuts could slow progress on rulemaking initiatives, including traceability requirements, contaminant action levels, and artificial dye bans (see below).
Companies would be wise to continue treating proactive food safety management as a strategic investment, not a compliance cost, and maintain federal-level food safety compliance readiness, regardless of inspections and enforcement.
Tip: Strengthen internal food safety management systems (FSMS) to prepare for potentially more strict state standards. Prioritize internal audit, environmental monitoring, supplier management and verification, and corrective action programs. Maintain compliance calendars and clear records and documentation.
FDA Regulatory Updates
As noted above, budget cuts are impacting finalization and implementation of several key FDA rules, several of which have been in progress for years.
Food Traceability Final Rule: FSMA 204
FDA’s Food Traceability Rule remains on everyone’s radar. Originally passed in November 2022 with a compliance date of January 20, 2026, that date has been delayed until July 20, 2028. At this point, FDA remains committed to implementing the full requirements of the final rule but giving industry additional time to comply. Traceability remains a huge concern given the number of recent high-profile recalls. Employing robust traceability processes is vital for reducing risks, regardless of regulatory requirements.
Tip: Develop records for capturing Key Data Elements (KDEs) associated with Critical Tracking Events (CTEs). Perform traceability exercises and mock recalls to help identify gaps in testing protocols, verification processes, corrective action implementation, and overall adequate traceability processes. Invest in a good technology solution that integrates with the FSMS to streamline the traceability process.
California AB 660 “Sell by” Ban
Open date labeling (e.g., “best if used by”, “sell by”, “expires on”, “freeze by”) is not currently mandated or standardized by federal law. However, the State of California has forged ahead with standardizing the use of open date labeling on food products. Beginning July 1, 2026, California AB 660 implements the first mandatory food date labeling for companies selling food products in California. Under the new law, food products will no longer be allowed to display confusing “sell-by” or “best before” dates. Instead, food companies will use “best if used by” or “best if frozen by” to indicate peak freshness/quality and “use by” or “freeze by” to indicate when a food item is no longer safe to eat. Anticipate other states to follow California’s lead.
Tip: Understand what the various open date labeling phrases mean before applying them to products. If you currently (or intend to) sell products in California, standardize open date labeling practices to meet the new requirements by the July 2026 compliance date.
Removing Artificial Food Dyes
In April 2025, the FDA announced a series of new measures to phase out all petroleum-based synthetic dyes from the food supply by the end of 2026, including FD&C Green No. 3, FD&C Red No. 40, FD&C Yellow No. 5, FD&C Yellow No. 6, FD&C Blue No. 1, and FD&C Blue No. 2. The FDA is planning to proceed without any statutory or regulatory changes, relying instead on the voluntary efforts of the food industry in complying with its mandates to remove the synthetic dyes as quickly as possible from the market.
Tip: Inventory existing products and their ingredients to determine which products use synthetic dyes. Reformulate affected products to use natural color alternatives and identify and approve new suppliers of alternative ingredients. Update label design, adjust ingredient panels, and modify any relevant nutritional claims.
Environmental Action Levels
Since 2020, the FDA has been making progress on establishing action levels for environmental contaminants (specifically arsenic, lead, cadmium, and mercury) to reduce dietary exposure as much as possible. The Closer to Zero Initiative has prioritized foods commonly eaten by babies and young children, and in 2025, the final Guidance for Lead in foods intended for babies and young children was released. Draft guidance for arsenic and cadmium was scheduled to be drafted in 2025; however, it has been delayed.
Tip: Integrate environmental contaminant testing for lead, arsenic, cadmium, and mercury into existing Environmental Monitoring Programs, prioritizing high-risk categories. Conduct risk assessments to determine which ingredients and products are most vulnerable to contamination. Review and strengthen supplier requirements to address environmental contaminants.
“Product of USA” Rule
Effective January 1, 2026, the U.S. Department of Agriculture’s (USDA) rule on how voluntary U.S. origin claims (e.g., Product of USA, Made in the USA) can be used on meat, poultry, and egg products became enforceable. Under the rule, companies may only use one of the claims if an animal is born, raised, slaughtered, and processed in the U.S. Note that these requirements differ for single-ingredient products vs. multi-ingredient products. Previously, imported animals/meats could use the claim if processed domestically. It is important to note that using these claims remain voluntary; however, establishments that choose to use them must maintain documentation proving U.S. origin.
Tip: If you choose to voluntarily make U.S. origin claims, ensure meat, poultry, and egg products meet the U.S. origin requirements; maintain documentation to substantiate claims; and include required descriptions on labels following updated USDA Food Safety and Inspection Services (FSIS) guidance.
Certification Updates
SQF Edition 10
Earlier in 2025, the Safe Quality Food Institute (SQFi) announced it would be releasing Edition 10 of its Safe Quality Food (SQF) Code to align with the latest Global Food Safety Initiative (GFSI) benchmarking criteria, updated regulatory requirements, and scientific changes. That release has been delayed until early March 2026 due to an extension in the GFSI benchmarking application timeline. The planned SQF Edition 10 changes focus on three critical areas: food safety culture, change management, and environmental monitoring. Pending completion of the review process, the first Edition 10 audits could begin as early as January 2027.
Tip: Assess current SQF program elements, identify improvements that are internally desirable and required by Edition 10, and implement those updates that will make the SQF program more useful to the business.
FSSC 22000 Version 7
Food Safety System Certification (FSSC) announced in October 2025 that it is commencing the development of FSSC 22000 Version 7. The new version will focus on incorporating the updated ISO 22002 prerequisite programs (PRPs), aligning with the GFSI benchmarking requirements, strengthening requirements to support UN Sustainable Development Goals (SDGs), and providing more structure for the division of food chain categories. Release of Version 7 is anticipated at the end of Q1 or beginning of Q2 2026.
Tip: Assess current FSSC 22000 program elements against potential changes. Review PRPs (e.g., sanitation, pest control, allergen management, waste handling) and update standard operating procedures (SOPs). Consider sustainability metrics, including energy, water, and waste use to ensure SDG alignment.
Others to Watch
Dietary Guidelines for Americans 2025-2030. HSS and the USDA have published new Dietary Guidelines with the underlying message to “Eat real food.” Changes include increased recommended daily protein intake, emphasis on dairy, and the avoidance of highly processed foods. Currently, “highly processed foods” or “ultra processed foods” are not clearly defined; however, there are efforts at the state and federal levels to define and regulate the category.
PFAS “Forever Chemicals”. There is currently a patchwork of federal requirements and state-level regulations and product bans in the U.S. related to per- and polyfluoroalkyl substances (PFAS). The FDA has been working to limit PFAS in food and food packaging; in 2024-2025, the agency also committed to testing foods from the general food supply to more accurately estimate exposure to PFAS from foods. While federal regulatory efforts have slowed significantly, organizations need to stay informed, especially at the state level, and address PFAS in their operations, products, supply chains, and waste streams accordingly.
Artificial Intelligence (AI). As noted above, budget cuts are prompting the FDA to accelerate the use of AI to streamline operations and improve efficiency. Many food companies are also starting to test the limits on what AI can—and can’t—do effectively and correctly. While AI may be leveraged as a tool to aggregate data, enhance traceability and recall readiness, automate routine tasks, and draft certain documents (e.g., audit reports, inspection summaries, training materials), using AI must be done thoughtfully, as it cannot replace food safety programs and human involvement. In fact, over‑automation without expert oversight can significantly increase error and legal exposure.
Looking Ahead
As we move into 2026, it will be important to monitor status of these trends and issues, as timelines may shift without warning and states may enact their own requirements. Start preparing now, even if deadlines have been pushed back—things could change. Now is the time to:
- Reassess regulatory exposure, strengthen internal systems, and ensure food safety programs are resilient, auditable, and aligned with broader business objectives.
- Implement proactive planning, disciplined data management, and thoughtful use of technology to turn uncertainty into a competitive advantage.
- Leverage partnerships with consulting partners with deep regulatory insight and practical implementation experience to help navigate the evolving regulatory landscape, protect against emerging risks, and position your organization to sustain whatever the next regulatory cycle brings.

Comments: No Comments
EHS Trends on the Horizon for 2026
As we move into 2026, the environmental, health, and safety (EHS) landscape is entering a period of heightened complexity and contradiction. Significant federal budget cuts and deregulation are reducing oversight from agencies like the Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA), while state enforcement, investor expectations, global regulations, and public scrutiny continue to intensify. At the same time, emerging risks and opportunities (e.g., PFAS, water scarcity, AI-driven systems, data center projects) are reshaping how organizations must think about compliance, risk, and resilience. Here’s what KTL currently has on our radar for 2026.
EPA Budget Cuts
The EPA’s FY2026 budget is a 54% reduction from the FY2025 budget and its lowest in approximately 50 years. Not unexpectedly, this lack of funding is sharply reducing EPA’s planned enforcement capacity in 2026 across all major statutes, focusing enforcement efforts on “clear and substantial violations.” Major funding cuts include criminal enforcement (- 49%), civil enforcement (- 30%), compliance monitoring and inspections (- 35%), and Environmental Justice (EJ) enforcement (100% eliminated).
Despite federal inspections and enforcement sharply declining in 2026, organizations remain fully liable under the Clean Air Act, Clean Water Act, RCRA, TSCA, EPCRA, and CERCLA, and major incidents will draw intense scrutiny. While it may be tempting to let internal environmental programs and systems slide due to less federal oversight, dismantling internal EHS and Environmental Social Governance (ESG) systems may create higher long‑term risks, particularly as many states expand enforcement to fill the federal gap.
Tip: Treat proactive environmental management as an asset protection tool, not a regulatory cost. Preserve internal audit, monitoring, and corrective action programs; keep permit tracking, compliance calendars, and incident response processes intact; and maintain clear records and documents.
EPA Deregulation
Consistent with its budget cuts, the EPA has significant deregulation planned for 2026 focused largely on rolling back key climate‑related rules, including Vehicle Emission Rules and the Clean Power Plan 2.0, which regulates fossil fuel-fired power plants. In addition, the following rules are all on the proverbial chopping block:
Federal Greenhouse Gas (GHG) Reporting
The EPA is proposing to largely end mandatory federal GHG reporting beginning in 2026. If finalized as proposed, most industries would no longer be required to report GHG emissions under the GHG Reporting Program (GHGRP), and oil and gas industry reporting under Subpart W would be paused until 2034, with limited exceptions. Though federal reporting may disappear (unless tied to a clear statutory mandate), many state programs still rely on GHGRP‑style data; SEC, investor, and customer pressure for emissions disclosure is expected to continue; and lenders and insurers continue requiring verified emissions data.
Tip: Organizations that stop internal tracking may increase long‑term risk, particularly if future administrations reinstate reporting. Maintain internal GHG inventories and related transparency processes, and archive methodologies so reporting can restart without rebuilding systems.
Risk Management Program (RMP) Rule
Although the EPA has not yet issued a final rule, the Agency is reconsidering the Risk Management Program (RMP) requirements related to cross-agency coordination in the chemical transportation industry. This may include narrowing coordination requirements to onsite emergencies only, no longer requiring facilities to report accidents occurring off site during transport, eliminating mandatory sharing of RMP data with transportation regulators, and removing implied responsibility for transporter training verification, carrier safety management practices, and emergency preparedness beyond the facility.
Core RMP applicability remains and onsite transfer operations (loading/unloading) will still fall under RMP. Importantly, Emergency Planning and Community Right-to-Know Act (EPCRA) emergency planning obligations remain unchanged, state RMP programs are unaffected, and related OSHA Process Safety Management (PSM) and Department of Transportation (DOT) HazMat rules still apply independently.
Tip: Refine your chemical transportation risk management approach, as necessary, by remapping regulatory responsibility to the correct agency. Maintain strong DOT, OSHA, EPCRA, and contractual controls; preserve emergency preparedness; and continue sharing site‑specific emergency data with responders. Track state rulemaking, especially if operating across multiple jurisdictions.
OSHA Budget Cuts
The FY 2026 Department of Labor budget reflects reduced funding for OSHA, with cuts affecting staffing and compliance assistance programs, while pushing the agency to streamline enforcement activities rather than expand them. The result is a leaner OSHA that is prioritizing high-impact enforcement for Severe Injuries and Fatalities (SIFs) vs. routine inspections and prevention-first support. With reduced OSHA staff who are focused largely on high-profile case work, day‑to‑day guidance from OSHA will become harder to obtain, and there will be less capacity for outreach, training, and informal guidance.
Budget cuts reduce OSHA capacity, but not an organization’s liability. Standards, citation authority, and penalty structures remain intact, and enforcement still applies after incidents. SIFs will require prompt response from companies and appropriate documentation and records.
Tip: Strengthen internal investigations, recordkeeping practices and systems, and incident response readiness. Leverage relationships with third parties to fill in the gaps for safety support, training, and guidance.
OSHA Streamlined Rulemaking
Despite budget pressure, OSHA will continue to work on streamlined rulemaking to promote the following:
Heat Illness Prevention
Heat Stress legislation is anticipated in 2026, which will require employers to develop and implement a formal, written program that explains how heat hazards are identified, controlled, and managed.
Tip: Develop or update a Heat Illness and Injury Prevention Plan (HIIPP) to meet proposed regulatory requirements, including hazard assessments, high exposure tasks/locations, controls, acclimatization procedures, roles and responsibilities, training, incident response, and recordkeeping.
Workplace Violence Prevention
While no federal legislation is anticipated, civil unrest in areas around the country is causing many states to consider creating their own requirements for employers to help prevent workplace violence and protect workers from inside or outside attackers.
Tip: Evaluate your Emergency Action Plan and update it to reflect expectations for workers when dealing with workplace violence incidents. Include leadership commitment, clear policies and procedures, training, and continuous improvement.
Recordkeeping and Transparency
There is talk of more stringent data collection and public availability of that data for incidents. This is currently a big unknown given OSHA’s limited resources; however, there are indications of AI-enabled systems being introduced to support incident reporting, data analysis, and public data availability.
Tip: Data accuracy matters more than ever, as reporting errors themselves may become enforcement triggers. Implement and maintain systems to accurately collect, manage, and report incident data.
Others to Watch
PFAS “Forever Chemicals”. Per- and polyfluoroalkyl substances (PFAS) remain a focal point, as does the uncertainty surrounding, their regulation. There is currently a patchwork of federal requirements and state-level regulations and product bans in the U.S. The Federal government is on the path to making U.S. rules less stringent, while global restrictions are accelerating. Currently, the reporting period for manufacturers and importers of PFAS under the Toxic Substances Control Act (TSCA) will begin on April 13, 2026, with a final deadline for most by October 13, 2026. The EPA also has a pending rule to designate nine specific PFAS compounds as hazardous constituents under the Resource Conservation and Recovery Act (RCRA), requiring corrective action for their release. Federal PFAS legislation is unlikely; however, keep an eye on state regulations and other bills for any provisions associated with PFAS, particularly in industries such as textiles, cleaning products, cosmetics, and cookware.
Water Use. Water use is becoming a more significant EHS concern, particularly in western states, due to rising demand, increasing scarcity, and the environmental impacts associated with over-extraction (see Data Centers below). Desalinization plants and using non-potable water for industrial use, golf courses, etc. can create potentially very negative environmental impacts on entire ecosystems. Make sure your environmental audits every consider things like energy and water conservation. Monitor state regulations, particularly where scarcity is a concern. Incorporate water conservation into ESG strategies to elevate its importance.
Artificial Intelligence (AI). AI is everywhere, and EHS is not excluded. EPA and OSHA staffing shortages are accelerating interest in using AI for automation. And organizations are testing the limits on what AI can—and can’t—do effectively and correctly. The assumption that AI can be used to automate and manage EHS programs is a slippery slope. While AI can be leveraged as a tool for things like data aggregation and draft report development, using AI must be done thoughtfully, as it cannot replace EHS programs and human involvement. In fact, over‑automation without expert oversight can significantly increase error and legal exposure. Use caution exploring where you can use AI for more basic tasks that can be automated, as not all processes lend themselves to AI.
Data Centers. There has been an explosion of growth in data centers. This expansion is rapidly increasing demand for energy, driving more fossil fuel pollution, straining water resources, using agricultural land, creating noise pollution, and raising electricity prices across the country. This is of particular concern in parts of the Midwest, Texas, and Western states. At the federal level, there is currently limited direct regulation specific to data centers. Oversight typically occurs through state water withdrawal permits, air permits for generators, and local zoning and land‑use approvals. It is important to understand how the increase in data centers may impact ESG goals, particularly related to water, power, noise, and land use.
Looking Ahead
Organizations that treat 2026 as a moment to scale back EHS efforts risk being caught unprepared. Reduced federal activity does not equate to reduced liability. In fact, given the uncertainty, proactive, well-integrated EHS programs will be increasingly critical for protecting people, assets, and long-term enterprise value. Now is the time to:
- Reassess regulatory exposure, strengthen internal systems, and ensure EHS programs are resilient, auditable, and aligned with broader business and ESG objectives.
- Implement proactive planning, disciplined data management, and thoughtful use of technology to turn uncertainty into a competitive advantage.
- Leverage partnerships with consulting partners with deep regulatory insight and practical implementation experience to help navigate the evolving regulatory landscape, protect against emerging risks, and position your organization to sustain whatever the next regulatory cycle brings.

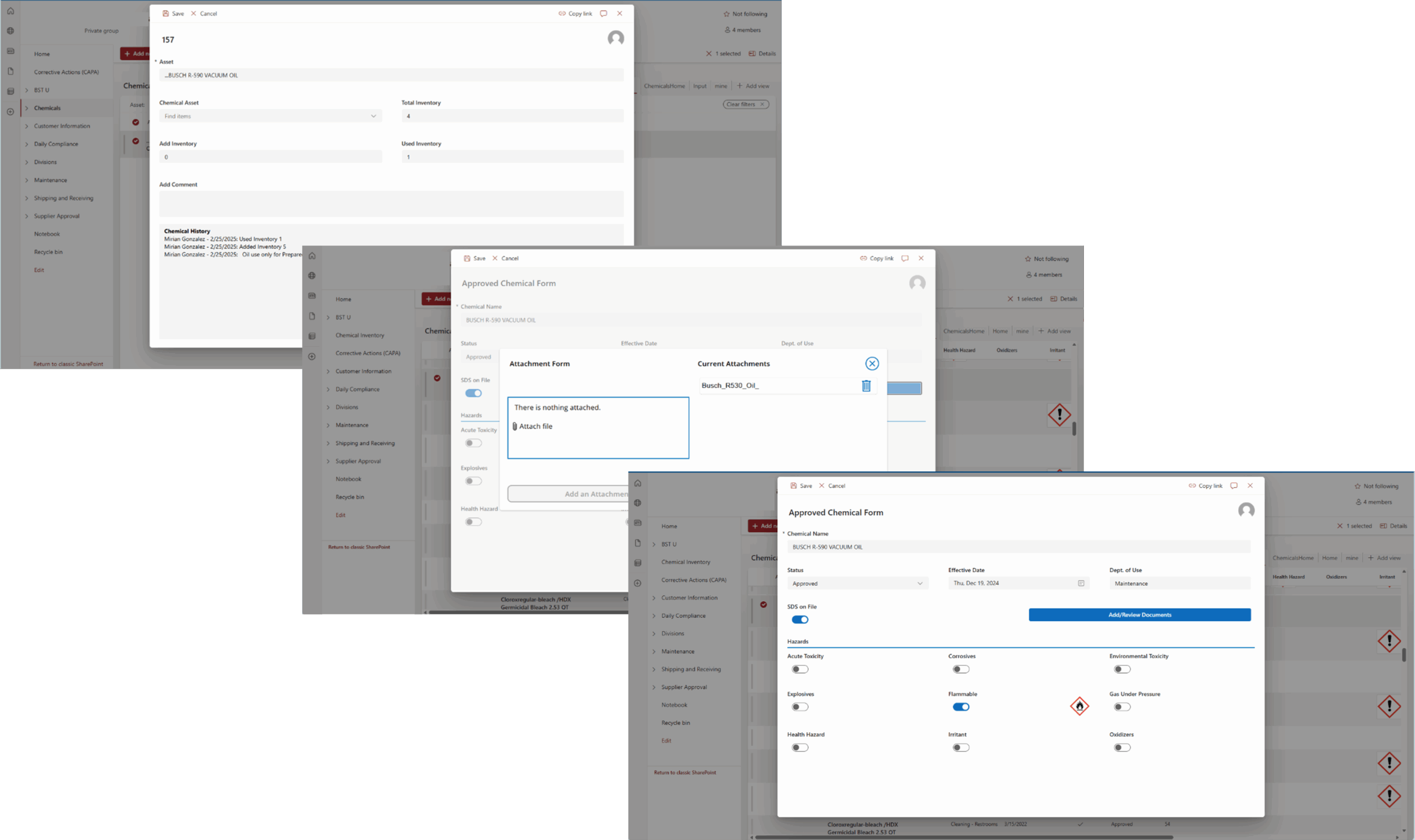
Tech Corner: Chemical Inventory Tool
Functionality: What does it do?
Chemical inventories are required for any organization using or managing hazardous chemicals in the workplace. They can be used to track inventory, communicate hazards, maintain Safety Data Sheets (SDS), and more. For companies using many chemicals, managing chemical quantities, historical usage, and associated documentation can be a challenge. KTL uses the Microsoft Power Platform to build and customize a Chemical Inventory Tool to replace scattered outdated systems, allowing organizations to more efficiently create inventories, track and manage chemicals, and meet regulatory compliance requirements.
Benefits: Why do you need it?
KTL’s Chemical Inventory Tool helps organizations using or managing chemicals to maintain compliance and streamline chemical management processes through:
- Managing Inventory: Maintains an accurate, up-to-date record of all chemicals onsite and their historical quantities.
- Centralizing Data Storage: Stores all chemical information in one location for easy access and consistency across sites.
- Communicating Hazards: Provides employees with quick access to chemical details, associated hazard information, and required personal protective equipment (PPE) for handling the chemical, reducing the risks of chemical handling and storage.
- Maintaining SDS: Connects chemical data to SDSs, helps ensure current versions are available, as required, and can store previous versions to meet the 30-year retention requirement.
- Streamlining Workflows: Creates workflows to manage chemical approvals, provide low supply reminders, and prompt regular chemical and SDS review.
- Reporting: Enables organizations to easily generate chemical inventories to comply with Tier III and Tier II reporting requirements and to provide to Local Emergency Planning Committees (LEPCs) and emergency response personnel.
Technology Used
- SharePoint or Dataverse
- Power Apps
- Power Automate

Comments: No Comments
SQF Edition 10 Delayed
Earlier in 2025, the Safe Quality Food Institute (SQFi) announced it would be releasing Edition 10 of its Safe Quality Food (SQF) Code to align with the latest Global Food Safety Initiative (GFSI) benchmarking criteria, updated regulatory requirements, and scientific changes. The publication date was targeted for September 2025, with audits beginning in April 2026. However, SQFi recently announced that the release of SQF Edition 10 has been delayed due to an extension in the GFSI benchmarking application timeline, which now runs through March 2026.
Because of this delay, SQF Edition 9 will remain the recognized version of the SQF Code. This means that audits will continue under the requirements of Edition 9 until Edition 10 is released. At that point, sites will have a six-month transition period before audits begin under Edition 10.
As KTL reported this summer, the SQF Edition 10 changes remain focused on three critical areas: food safety culture, change management, and environmental monitoring. SQFi has reinforced that, when released, the Edition 10 changes will reflect the industry’s growing understanding that food safety is not just about compliance, but about creating a holistic, proactive approach to protecting consumers.
With the additional time before the release and implementation of Edition 10, now is an ideal time for facilities who are certified to SQF to assess current SQF program elements, identify improvements that are internally desirable and required by Edition 10, and implement those updates that will make the SQF program more useful to the business.

Kestrel Tellevate News / Safety
Comments: No Comments
KTL Safety Culture Article Featured in ASSP’s Professional Safety Journal
An organization’s safety culture is ultimately reflected in the way safety is managed in the workplace. Strong safety cultures have a number of key attributes in common that promote responsible safety and health practices and beliefs across the organization. When these characteristics come together, everyone wins. Benefits that can be expected include:
- Fewer incidents, losses and disruptions
- Improved employee morale
- Increased productivity
- Lower workers’ compensation and insurance claims
- Improved compliance with OSHA regulations and state occupational safety and health programs
- Improved reputation to help attract new or retain existing customers and employees
- Better brand and shareholder value
A safety culture action plan outlines the tools and strategies organizations can use to improve their existing safety culture and ensure that it is integrated with overall business goals, vision, and mission.
Read KTL’s full article, Creating a Safety Culture Action Plan, authored by KTL Senior Associates April Greene, CSP, CHMM, and Will Brokaw, MA, as published in the November 2025 issue of ASSP’s Professional Safety Journal.

Tech Corner Q&A: Using the Software You Have
Having a simple, centralized compliance information management system to manage, track, communicate, and report compliance program information can enable staff to complete required tasks, improve compliance performance, and support operational decision-making. It sounds expensive, but it doesn’t have to be—not when most companies already have the software and just need the right combination of information technology (IT) and quality, food safety, and/or environmental, health, and safety (EHS) expertise to customize it.
We recently sat down with KTL’s Power Platform Team to talk about how organizations can leverage Microsoft 365 and the Power Platform to collect, track, report, and manage compliance; reduce risk; and improve operational efficiency.
Q: First, what is Microsoft 365 and the Power Platform?
Microsoft 365 is a suite of tools built to drive productivity across your organization. If you use Outlook or Office tools like Word, Excel, and SharePoint, then you are already using Microsoft 365. The Power Platform (i.e., Power Apps, Power Automate, Power BI) is an extension of Microsoft 365 that allows users to build and deploy custom business applications, workflows, and dashboards.
Q: Why Microsoft 365 and the Power Platform?
The Microsoft 365 and Power Platform suite of tools can be adapted and designed to manage documents and records, capture data, and provide program reporting to support and meet overall business and compliance needs. The best part? When developing Microsoft 365-based systems, we are not only leveraging software people are already familiar with, but we are also working with existing Microsoft licenses for development. Often, users also have existing licenses that allow them to use the developed systems. This helps keep budgets in check and fosters easy adoption and buy-in.
Q: What does the process look like?
No two projects are exactly alike. The process will vary a bit depending on your company’s current state and needs, but generally, most projects follow three phases:
- Planning: In many cases, companies know exactly what tools they want. In these cases, we meet with team members to get a solid understanding of needs and requirements and then build a scope that meets objectives. Sometimes, the needs aren’t as well defined. In these situations, we start by conducting an assessment to review current information management processes, practices, and tools; help the company set priorities; and develop a development plan that meets identified needs.
- Development, Review, and Rework: We take an iterative and collaborative approach to our development process that emphasizes customer input and feedback. This includes building out tools to a functional point and then requesting feedback to help ensure those tools meet requirements and deliver value.
- Turnover and Continued Support: Once development is complete, turnover is often as simple as setting a date for staff to start using and managing the tools. We conduct training that focuses on the basics of how to use the tools we develop. In addition, we offer continued support to make adjustments that may be needed once you start using the tools.
We help set priorities based on ease of implementation, compliance risk, business improvement, and value to your company. We often recommend starting with something simple—perhaps creating a document library or converting a paper checklist into an electronic form. We can work at your pace and budget to build and integrate only the modules you need one at a time or as a fully functional system. Our goal is to develop the system in a way that will work with your business and encourage buy-in, rather than you having to adapt your business to off-the-shelf software requirements and capabilities.
Q: From a technical standpoint, what will my IT department want to know if we already use Microsoft 365—or if we don’t?
If you already use Microsoft 365, you already have the accounts needed to use most systems KTL builds. Our developers just need access to your tenant to begin building. If you aren’t currently using Microsoft 365 or have not been using SharePoint, KTL will work with your IT department to set up the appropriate infrastructure for the systems we build. This typically involves selecting licensing, establishing a domain, and assigning licenses.
After access is established, our team can begin developing and piloting your system. Once users are comfortable with the applications, we hand the system over to you. The system and information are all yours. All applications run on Microsoft servers, and there are no external or proprietary software requirements. We train your staff to manage the system going forward, so minimal ongoing IT department support is needed.
Q: What are the benefits associated with creating Microsoft 365 system?
Microsoft 365 offers a unified and familiar platform that simplifies access and is adaptable to evolving data management and reporting needs. This allows you to maximize efficiency and connectivity between various operational and corporate groups without the added cost of separate tools. With Microsoft 365 you get:
- Smarter Data and Document Management: Replace paper systems, simplify data entry, and build flexible databases. Upload, organize, and retrieve records with ease.
- Streamlined Communication and Collaboration: Share information, send/receive notifications, and connect teams across departments, sites, and facilities.
- Compliance and Operational Oversight: Manage regulatory tasks, prepare for inspections, and assign role-based permissions to ensure accountability.
- Insightful Reporting and Continuous Improvement: Consolidate reporting, access real-time dashboards, monitor performance, and capture institutional knowledge for long-term sustainability.
Q: What makes KTL’s approach to information management different?
KTL is not a software company. Rather, we adapt Microsoft 365 and the Power Platform to your compliance needs. Our team is made up of EHS, quality, and food safety professionals with the technology expertise to design and build systems that allow you efficiently and effectively manage compliance and business processes. Because we understand regulatory, certification, and operational requirements, we are better able to make sure our technology solutions support your objectives.
Many companies look at software as a silver bullet—a fix for everything. But applying technology to operations isn’t about just finding and buying a software tool. It is about understanding business needs, then adapting and integrating the appropriate tool into existing operations. That means we start with the end user and build around that. If the system doesn’t work for the end user, then it’s not going to be used, no matter how fancy or how many bells and whistles it has.

Comments: No Comments
Gluten Added to Major Allergen Verification
The U.S. Department of Agriculture – Food Safety Inspection Service’s (USDA-FSIS) directive, Ongoing Verification of Product Formulation and Labeling Targeting the Nine Most Common (“Big 9”) Food Allergens, provides instructions to inspection program personnel (IPP) to verify that establishments are controlling and labeling the Big 9 food allergens in meat, poultry, and egg products. On September 11, 2025, USDA-FSIS reissued the directive to include gluten to be verified as an ingredient of public health concern.*
Why Gluten?
Gluten is a protein found naturally in wheat, rye, and barley. Many products contain gluten. According to the USDA, if meat, poultry, or egg products contain any of the following, they likely contain rye or barley and, subsequently, gluten:
- Malt extract
- Malt vinegar
- Rye flour
- Whiskey
- Malt flavoring
- Brewer’s yeast
- Barley flour
- Beer
- Malt syrup
- Yeast extract
- Soups
- Malted milk
- Spent grains
- Brown rice syrup
The National Institute of Health (NIH) estimates there are approximately two million Americans and one percent of the population worldwide who have celiac disease, an auto-immune disease triggered by consuming gluten-containing foods—plus many more with gluten sensitivities and intolerances. As such, the USDA considers gluten an ingredient of public health concern; however, it is not designated a Big 9 major food allergen (though wheat is). Even so, the updated USDA-FSIS directive now requires IPP to verify that establishments are accurately controlling and labeling gluten similarly to the Big 9 allergens.
Formulation Verification
The USDA-FSIS conducted analyses of voluntary recalls connected to undeclared allergens and determined the following:
- Many voluntary recalls occurred due to changes in ingredient suppliers, products in the wrong package or with misprinted labels, or changes to the product or ingredient formulation.
- Establishments failed to include allergens on the product label when product came into contact with an undeclared allergenic ingredient that was not directly added to the product (i.e., unintentional adulteration of product).
When an establishment allows product to enter commerce with undeclared allergens, it signifies that the food safety system has failed in some way to control the hazards associated with the allergens. As such, the purpose of the Big 9 Formulation Verification task is to verify that establishments are properly labeling the Big 9 major food allergens and gluten when included in the formulation of a product to avoid these issues.
During the Big 9 Formulation Verification task, IPP verify the following:
- The establishment has developed and implemented preventive or control measures in its Hazard Analysis and Critical Control Points (HACCP) Plan, Sanitation Standard Operating Procedures (SSOPs), and/or other prerequisite programs (PRPs) to address allergens.
- All ingredients used in the production of the product are present on the product formulation record.
- All ingredients in the product formulation are declared in the ingredient statement on the product label by common name in descending order of predominance.
- All ingredients listed on the labels of incoming food and food ingredients are also listed on the labels of the products in which they are used.
- All ingredients listed in a “may contain” or “produced in a facility” statement on incoming food and food ingredients are also listed on the final product label, except when the establishment contacts the supplier for further clarification and addresses the statement in the hazard analysis.
- The appropriate label is applied to the product.
- The applied label is consistent with the establishment’s label approval on file.
If IPP find that a product contains one of the Big 9 allergens or gluten that is not declared on the final label, they document a food safety labeling noncompliance in a Noncompliance Record (NR).
The Big 9 Formulation Verification task is conducted monthly; however, frequency may increase if there are indicators of an increased risk related to the Big 9 allergens, gluten, ingredients of public health concern, or other undeclared ingredients linked to the establishment, including the following:
- Public Health Alert (PHA) or recall.
- Consumer complaints.
- History of sanitation and/or HACCP NRs.
- History of labeling NRs.
- Recent product formulation changes, adjustments to ingredients, supplier changes, or new products added by the establishment.
Questions to Ask
The USDA-FSIS cautions that these updates do not change the fact that all ingredients, other than processing aids, whether allergens or not, must be declared on the label.
If you produce meat, poultry, and/or egg products, it is important to understand what allergens—and now gluten—you have at your facility, how you use them in product formulation, and how you label them as ingredients in your products. The USDA-FSIS outlines the following questions for the Big 9 Formulation Verification task that you can ask internally to ensure your products comply with labeling requirements:
- Which of the Big 9 allergens and/or gluten are included in products you produce at your facility?
- Does your facility use any components that have multiple ingredients that you do not produce or mix onsite?
- For the selected product, do you have any labels that claim the absence of one or more of the Big 9 allergens and/or gluten?
- For the selected product, do you implement control measures within your HACCP Plan, SSOPs, PRPs, or other programs to prevent misbranding (e.g., mistakes on labeling)?
- For the selected product, do you implement control measures within your HACCP Plan, SSOPs, PRPs, or other programs to prevent allergen cross-contact (e.g., food allergens from one product getting into another product that doesn’t contain the allergen)?
* Note: USDA-FSIS is also removing certain tree nuts that are no longer considered major food allergens from the directive, updating the milk category to include milk from other ruminant animals (e.g., sheep, goats), and updating the eggs category to include eggs from other fowl (e.g., duck, quail), in accordance with the Food and Drug Administration’s (FDA) January 2025 updates to food allergen labeling requirements.

KTL to Present on Human Health Risk Assessments
KTL will be joining the technical program of the Society of Environmental Toxicology and Chemistry (SETAC) North America 46th Annual Meeting November 16-20, 2025 in Portland, Oregon. SETAC is dedicated to advancing environmental science and science-informed decision-making through collaboration, communication, eductaion, and leadership.
KTL Senior Associate Margaret Roy will be presenting Representation of the Homeless in Human Health Risk Assessment on Monday, November 17 at 10:00 am as part of Session 5.08: Beyond Direct Contact: Non-Traditional Exposure Scenarios in Human Health Risk Assessment.
Human Health Risk Assessments (HHRAs) are used to assess contaminant exposure and risk to defined populations, such as residents, commercial workers, and construction workers. HHRAs can also be used to assess risk for the homeless population. This presentation will discuss some of the challenges associated with developing an exposure model to represent the homeless sheltering and living on a contaminated site. For example, interviews with individuals may be necessary to develop an accurate exposure model. The presentation will also discuss the importance of the field sampling crew, from observing field conditions to safety concerns.
