Wasted food makes up the largest percentage—over 20%—of any one material sent to landfills and incinerators each year in the U.S. This large volume of disposed food is a main contributor to total U.S. methane emissions, a greenhouse gas (GHG) with 21 times the warming potential of carbon dioxide. Financially, wasted food costs America more than $100 billion annually from disposal costs of municipal waste management, over-purchasing costs, and cost of lost energy. And all this wasted food is happening when nearly 40 million Americans are food insecure.
In short, wasting food impacts the sustainability of our economy, our society, and the environment—also known as the triple bottom line of sustainability. But through sustainable food management, it is possible to help businesses and consumers save money, create outlets for those in our communities who do not have enough to eat, and conserve resources for future generations.
Wasted Food and Food Loss
The U.S. Environmental Protection Agency (EPA) uses the term wasted food instead of food waste to describe food that is not used for its intended purpose. This terminology conveys the notion that a valuable resource is being squandered. The U.S. Department of Agriculture’s (USDA) Economic Research Service (ERS) further defines food loss as the “edible amount of food, postharvest, that is available for human consumption but is not consumed for any reason.”
Food loss occurs at every stage of the supply chain—from farm to table. How that loss is managed plays a vital role in how it impacts our society. That is where sustainable food management comes to play.
What Is Sustainable Food Management?
According to EPA, sustainable management of food is “a systematic approach that seeks to reduce wasted food and its associated impacts over the entire lifecycle, starting with the use of natural resources, manufacturing, sales, and consumption, and ending with decisions on recovery of final disposal.”
Sustainable food management is essentially a subset of sustainable materials management (SMM). As with SMM, the best approach to reducing food loss and waste is to not create it in the first place. Source reduction is the most effective way of reducing the environmental and financial impacts of wasted food and packaging because it prevents unneeded materials from ever being created. To do so is a process that involves performing a food waste assessment—much like an SMM lifecycle analysis (LCA)—to identify what and how much food (and food packaging) is being wasted.
A thorough food and packaging assessment serves as the foundation for reduction efforts. Having this general understanding can help identify appropriate strategies to avoid waste, cut down on disposal costs, reduce over-purchasing and labor costs, reduce water and energy use associated with food production, and reduce GHG emissions.
Based on the outcomes of the food waste assessment, EPA suggests some common strategies for reducing wasted food and packaging, which may include the following:
- Adjusting food purchasing policies to reduce excess food purchasing (i.e., use just-in-time purchasing, purchase items in bulk
 to reduce packaging).
to reduce packaging). - Storing and organizing food properly to reduce spoilage.
- Repurposing leftover food following food safety guidelines.
- Reducing to-go/takeout item packaging and using compostable/recyclable packaging.
- Reducing portion size of regularly wasted items.
- Using a system to identify over-purchased food items and to track wasted food.
- Continuously training staff on basic steps to minimize food waste (e.g., cooking and food preparing to reduce wasted food, plating practices).
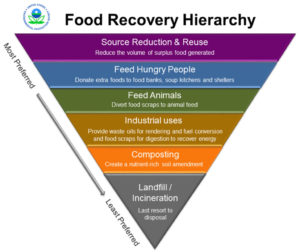
If excess food is unavoidable—and sometimes it is—reusing leftover food is possible as long as food safety guidelines are followed. The food can also be recovered to donate to hunger relief organizations to feed people in need. Even inedible food can be recycled into other products such as animal feed, compost and worm castings, bioenergy, bioplastics, and clothing. EPA’s Food Recovery Hierarchy identifies and prioritizes the actions organizations can take to prevent and divert wasted food. The top levels of the hierarchy are the most desirable alternatives because they create the most benefits for the environment, society, and the economy.
Part 4 of KTL’s series on Creating Sustainable Impacts will dive into some of the incentives and drivers for participating in sustainable food management.

