
Comments: No Comments
EPA Regulatory Alert: Requirements Increase for PFAS
In 2021, the Environmental Protection Agency (EPA) published the PFAS Strategic Roadmap to confront the human health and environmental risks of per- and polyfluoroalkyl substances (PFAS), also known as “forever chemicals” due to their ability to persist and bioaccumulate in the environment and human body. Through the Roadmap, the Agency has committed to making significant contributions in research and regulations to address concerns related to PFAS exposure. This has included recent actions to improve reporting on PFAS (October 2023), as well as adding seven additional PFAS to the Toxics Release Inventory (TRI) (January 2024).
In April 2024, EPA announced three more notable actions to tackle PFAS contamination.
National Drinking Water Standard
On April 10, 2024, EPA issued the first national, legally enforceable drinking water standard to protect communities from exposure to several PFAS known to occur individually and as mixtures in drinking water. The Safe Drinking Water Act (SDWA) requires EPA to set goals, known as Maximum Contaminant Level Goals (MCLGs), based only on health data and the potential impacts to public. MCLGs are not regulatory levels and are not enforceable. EPA then sets the enforceable Maximum Contaminant Level (MCL) as the highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. The MCLs, which are used for compliance determination, are set at specific concentrations that laboratories nationwide can measure with high certainty.
| PFAS | MCL | MCLG |
| Perfluorooctanoic acid (PFOA) | 4.0 ppt* | 0 |
| Perfluorooctane sulfonic acid (PFOS) | 4.0 ppt | 0 |
| Perfluorononanoic acid (PFNA) | 10 ppt | 10 ppt |
| Perfluorohexane sulfonic acid (PFHxS) | 10 ppt | 10 ppt |
| Hexafluoropropylene oxide dimer acid (HFPO-DA, aka “GenX Chemicals”) | 10 ppt | 10 ppt |
| Mixtures of any two or more of PFNA, PFHxS, perfluorobutane sulfonic acid (PFBS), and GenX Chemicals | Hazard Index** (unitless) | Hazard Index** (unitless) |
** The Hazard Index is a long-established approach that EPA regularly uses to determine the health concerns associated with exposure to chemical mixtures. EPA’s Hazard Index MCL is set at 1.
Under the standard, initial PFAS monitoring requirements mandate larger systems (i.e., serving more than 10,000 people) take four quarterly samples, while smaller systems (i.e., serving fewer than 10,000 people) take biannual samples. All public water systems have three years to complete initial monitoring for these chemicals and inform the public of the PFAS levels measured in the drinking water. Where PFAS exceeds the MCL, systems must start implementing solutions to reduce PFAS in their drinking water within five years. Drinking water systems have the flexibility to determine the best solution for their community based on a range of available approaches (e.g., granular activated carbon, reverse osmosis, and ion exchange system).
EPA estimates 6-10% of the 66,000 public drinking water systems subject to this rule may need to take action to reduce PFAS to meet the standards. As such, the Agency also announced nearly $1 billion in newly funding available through the Bipartisan Infrastructure Law to help states and territories implement PFAS testing and treatment at public water systems.
Now that EPA has finalized MCLs, states that have already established enforceable drinking water standards for PFAS (e.g., Maine, Massachusetts, Michigan, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Washington, and Wisconsin) must adhere to the federal standards if they are stricter or adopt new drinking water standards that are at least as stringent as the federal standards.
The new Rule is expected to put increased pressure on industry to reduce the presence of upstream PFAS contamination, eliminating it before it reaches the drinking water supply.
Superfund Designations
On April 19, 2024, EPA designated two widely used PFAS chemicals—PFOA and PFOS and their salts and structural isomers—as hazardous substances under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA, aka Superfund). This designation will allow EPA to address more contaminated sites, take earlier action, expedite cleanup, and ensure the polluter pays for the costs to clean up PFAS contamination.
Under the Rule, entities are required to report releases of PFOA and PFOS that meet or exceed the reportable quantity of one pound within a 24-hour period to the National Response Center, as well as state, tribal, and local emergency responders. As a discretionary statute, CERCLA affords EPA the discretion to then decide how to respond to a release based on whether it poses unacceptable risk to human health or the environment. It allows EPA to use one of its strongest enforcement tools to make polluters pay for investigations and cleanup rather than taxpayers through the Superfund Trust Fund.
The final Rule also requires federal entities that transfer or sell their property to provide notice about the storage, release, or disposal of PFOA or PFOS and guarantee (through a commitment in the deed) that contamination has been cleaned up or, if needed, that additional cleanup will occur in the future.
EPA is also issuing a separate CERCLA enforcement discretion policy to clarify that the Agency will focus enforcement on parties that have manufactured PFAS or used PFAS in the manufacturing process, federal facilities, and other industrial parties. It is not the Agency’s intent to pursue other parties, such as farmers, municipal landfills, water utilities, municipal airports, and local fire departments.
The Superfund designation is especially important, as delays in addressing contamination may worsen contamination as PFOA and PFOS have more time to migrate into water and soil. The designation may also encourage better waste management and/or treatment practices by facilities handling PFOA or PFOS to minimize releases, reduce CERCLA notification requirements, and mitigate liability risk.
This Rule puts PFOS and PFAS on equal ground with other hazardous substances regulated under Superfund. The addition will likely increase scrutiny of properties for potential PFAS releases that may impact negotiations and property values and result in liability for property owners. In addition, Section 306 of CERCLA further requires the Department of Transportation to list and regulate these substances as hazardous materials under the Hazardous Materials Transportation Act.
Destruction and Disposal
Finally, EPA has issued updated Interim Guidance on the Destruction and Disposal of PFAS and Materials Containing PFAS. The document offers guidance and recommendations for managers of PFAS and PFAS-containing materials to protect human health and the environment, including a new technology evaluation framework to help analyze the safety and effectiveness for new destruction and disposal (D&D) technologies.
In the Guidance, EPA encourages the use of D&D options that have a lower potential for releasing PFAS into the environment. It does not establish requirements for D&D of PFAS materials. The Guidance focuses on the following:
- Underground injection (UIC): Permitted Class I non-hazardous industrial or hazardous waste injection wells are designed to isolate liquid waste deep below the land surface to protect underground drinking water sources.
- Landfills: Permitted hazardous waste landfills are recommended when PFAS concentration is relatively high. Hazardous waste landfills have leachate emission protections to help control the environmental release of PFAS, which are important for PFAS-containing materials that may break down more easily in landfills.
- Thermal treatment: Permitted hazardous waste combustors and granular activated carbon reactivation units operating under certain conditions can destroy PFAS, while minimizing releases and exposures; however, some uncertainties remain.
- Interim storage with controls: While not a D&D technology, storage may be a short-term alternative for some PFAS materials, such as containerized or high PFAS-content materials. With proper controls, interim storage can control PFAS migration.
The Guidance encourages testing a range of methods at thermal treatment facilities before accepting large quantities of PFAS-containing materials. EPA has also released a new analytical test method (OTM-50) to help collect more data and answer questions.
More to Come…
Federal and state initiatives and regulations to manage PFAS are rapidly growing. KTL does not see the challenges associated with PFAS going away any time soon. If anything, we anticipate more facilities will be directly impacted by mitigation efforts and regulatory action to support EPA’s PFAS Strategic Roadmap, such as those outlined above.
It is important for facilities to have a good understanding of PFAS and PFAS-containing chemicals used onsite. Implementing a chemical inventory management system that documents and manages PFAS data can help ensure the facility meets these new requirements. Proper usage and disposal strategies, a comprehensive environmental management system (EMS), and a forward-thinking Emergency Response Plan will also remain vital tools for companies potentially dealing with PFAS to effectively manage the associated risks.

Tech Corner: Document Approval Process
Functionality: What does it do?
The documents an organization works with are subject to various internal policies, industry standards, compliance requirements, and more. As such, they often require review and approval by various stakeholders at various stages of development. A document approval process uses workflows to streamline and standardize document approvals. KTL’s approval process is set up to automatically route documents, assign review tasks, track progress, and send reminders and notifications when needed. It takes documents from development through final approval, ensuring documents are reviewed at the appropriate time by the appropriate party.
Benefits: Why do you need it?
KTL’s document approval process provides the following:
- Document standardization.
- Streamlined approach for approving and completing tasks involving documents.
- Improved document integrity and accuracy with less opportunity for human error.
- Business efficiencies from automated and streamlined document handling practices.
- Version control and history to create a reliable audit trail.
- Improved access/security for sensitive documents.
Technology Used
- Microsoft SharePoint
- Microsoft Power Automate

Comments: No Comments
Plastics Recycling for Food-Contact Materials
According to the United Nations Environment Programme (UNEP), we produce about 400 million tons of plastic waste every year globally. Approximately 36% of that plastic is used in packaging, including single-use plastic products for food and beverage containers.
The European Commission estimates that approximately 50% of plastic packaging in the European Union (EU) is used for the food and beverage industry. In the U.S., food packaging comprises approximately two-thirds of all packaging material produced, and correspondingly, the U.S. Environmental Protection Agency (EPA) cites that food and food packaging materials make up almost half of all municipal solid waste.
Food packaging is used to help make food clean, safe, transportable, and shelf stable. Unfortunately, most of it is single use and is not recycled or reused. But there is a growing trend throughout the U.S., as well as in Canada and the EU, to increase the use of post-consumer recycled (PCR) materials in packaging products, including plastic.
Regulatory Background
California enacted the first minimum recycled content laws in the U.S. in 1990 for fiberglass, glass containers, plastic containers, and plastic trash bags. Fast forward to late 2023, and the Association of Plastics Recyclers (APR) indicates that seven states are currently in various stages of developing and/or implementing state minimum recycled content laws, including California, Maine, Washington, New Jersey, Connecticut, Maryland, and New York.
In general, these laws establish incremental minimum recycled content requirements for various packaging materials and associated timelines for compliance. Impacted products include plastic beverage bottles, plastic carryout bags, trash bags, household cleaning and personal care products, and others. Some laws allow for averaging recycled content across a company’s covered products portfolio to meet requirements. Some states require third-party certification to ensure accountability.
The New Jersey Recycled Content Law, which recently came into effect on January 18, 2024, is considered one of the most ambitious recycled content laws to date, as it goes beyond traditional rules to cover rigid plastic containers.
Given current and potential upcoming legislation, manufacturers and retailers alike must be aware of which products are covered by recycled content standards, as there may be restrictions on the use and sale of products that do not comply.
Plastic Recycling for Food-Contact Materials
According to the Food and Drug Administration (FDA), there are only a few major varieties of plastic that meet the Agency’s guidelines for safe contact with food and that can be considered food grade and FDA-compliant. To be FDA-compliant, packaging must be able to withstand whatever environment it will be used in (e.g., extremely hot oven for cooking, freezing temperatures, and cleaning and sanitization). The packaging material must also be compatible with the type of food it will be in contact with and must not leach any chemicals if the food is acidic or has high moisture content.
The following plastics are generally considered safe for food:
- High-density polyethylene (HDPE), the most common household plastic, is used to make beverage bottles, butter containers, cereal box liners, and thicker food storage buckets. FDA evaluates the use of recycled HDPE on a case-by-case basis, as it can become unsafe in the recycling process.
- Low-density polyethylene (LDPE) is similar to HDPE but less rigid. It is used for products like squeeze bottles, plastic wrap, six-pack rings, etc. While LDPE is chemical-resistant and does not leach, recycled LDPE is not deemed safe for food contact.
- Polycarbonate resin (PCR) has presented some concerns about the presence of bisphenol A (BPA); however, FDA studies conclude that intake from BPA in plastic is very low and does not have apparent negative health impacts.
- Polypropylene (PP) is most often used for single-serve containers (e.g., yogurt, cottage cheese) and reusable food storage containers. It is microwave safe and nonvolatile.
- Polyethylene Terephthalate (PET) is used to make any plastic jars and beverage containers (e.g., 2-liter bottles, peanut butter jars, salad dressing containers, etc.). It repels microorganisms and does not corrode.
While many of these plastics are only FDA-compliant and food safe in their unrecycled state, recycled PET is an FDA-approved plastic for food contact. Most plastic food packaging that is not made of PET cannot be recycled into new food packaging due to missing processes (i.e., waste collection, separation, decontamination) and other safety concerns. These other types of plastic food packaging are typically only downcycled or not recycled at all.
Recycling Responsibilities
Manufacturers of food-contact packaging made from recycled plastic are responsible for ensuring that recycled material meets the same specifications for virgin material, as outlined in 21 CFR parts 174-179. The regulations state that any substance used as a component of articles that contact food shall be of a purity suitable for its intended use.
Because recycled food-contact materials have content that is recovered from waste, it may be contaminated with substances originating from previous use or other waste—and that contamination may end up in our food and be harmful to human health. The FDA cites the following main safety concerns with the use of PCR plastic materials in food contact articles:
- Contaminants from the PCR material may appear in the final food-contact product made from the recycled material.
- PCR material that may not be regulated for food-contact use may be incorporated into food-contact material.
- Adjuvants in the PCR plastic may not comply with the regulations for food contact use.
Although not required by law, recyclers of plastic intended for food-contact use may submit information on their recycling process to FDA for evaluation. In turn, FDA will consider each proposed use of recycled plastic on a case-by-case basis and issue informal advice as to whether the recycling process is expected to produce food grade, FDA-compliant PCR plastic based on the following:
- Complete description of the recycling process, including a description of the source of the PCR plastic, any source controls in place, and a description of steps taken to ensure the recyclable plastic is not contaminated at any point.
- Results of any tests performed to show that the recycling process removes possible incidental contaminants.
- Description of the proposed conditions of use of the plastic (e.g., temperature, type of food, duration of contact, repeated or single use).
Recycling plastic food packaging is widely seen as an opportunity to reduce our environmental impacts across the globe. While plastic food packaging recycling is limited extent due to material properties, waste management processes, and chemical safety concerns, legislation is pushing recycling forward where it is safe. Given this, manufacturers and retailers must be aware of which products are covered by recycled content standards and make plans to meet established timelines for minimum recycled content requirements to avoid restrictions on the use and sale of products that do not comply.

Tech Corner: GHG Inventory and Reporting Tool
Functionality: What does it do?
According to the Environmental Protection Agency (EPA), a greenhouse gas (GHG) inventory is a list of emission sources with the associated emissions quantified using standardized methods. KTL’s GHG inventory and reporting tool helps to organize energy use and GHG information and responsibilities. The tool factors in all fuel sources across an organization, including direct (from the facility), direct mobile, and indirect facility combustion, as well as hours worked so that all calculations can be normalized. The tool has built-in conversion factors for reported figures (so facilities report data in their original units) to metric tons of CO2e emitted, allowing the organization to track and understand GHG emissions across the organization.
Benefits: Why do you need it?
KTL’s GHG inventory and reporting tool helps organizations to:
- Collect, track, and manage GHG emission source data and emission trends over time.
- Identify GHG and energy usage reduction opportunities and manage related risks.
- Establish the GHG tracking and documentation that is essential to comply with voluntary GHG programs or mandatory GHG reporting requirements, including the new SEC climate-related disclosure requirements.
- Measure progress towards local, regional, state, federal, and international climate goals.
- Create a baseline of GHG data to inform the development of Climate Action Plans.
- Evaluate the impacts of potential plant renovations, production changes, etc. on emissions.
Technology Used
- Microsoft SharePoint
- Microsoft Power Platform

KTL to Present at MBAA District Carolinas Technical Conference
KTL will be joining industry experts at the 2024 Master Brewers Association of America’s (MBAA) District Carolina Technical Conference to discuss food safety and regulatory implications of producing nonalcoholic and low-alcohol beer (NALAB).
MBAA District Carolinas 2024 Spring Technical Conference
May 4, 2024
Hopfly Brewing Company | Charlotte, NC
KTL Presentation: Food Safety and Regulatory Implications of Producing Nonalcoholic and Low-Alcohol Beer (NALAB)
The demand for NALAB has increased significantly in the past few years and is projected to continue. KTL’s presentation will describe the complexities associated with adding a NALAB beverage to a product portfolio and break down the requirements and considerations into actionable pieces. It will also discuss relevant food safety risks in traditional beer, such as allergen cross-contamination, chemical inclusion, and foreign object inclusion. Specifically, the presentation will cover the following:
- Interpretation and breakdown of the regulatory requirements for NALAB products.
- Differences in FDA regulatory requirements for traditional beer versus NALAB products.
- Known or reasonably foreseeable food safety hazards associated with traditional beer and NALAB products.
- Strategies that brewers can take to minimize food safety hazards.

KTL Expands Food Safety Resources
KTL is pleased to welcome the following new food safety resources to our team!

Samantha Humphrey, Consultant
Sam has 10 years of experience working in the food and beverage industry. She has specialized expertise in food safety training; regulatory compliance, particularly the FDA Produce Safety Rule; GMPs and GAPs; SOP development and implementation; GFSI certification; supply chain management; environmental monitoring; and food microbiology. Sam is a PCQI and Produce Safety Rule Lead Trainer, Certified ATD Master Trainer, Certified Professional – Food Safety (CP-FS), and Juice and Seafood HACCP certified. She is based in Raleigh, NC. Read her full bio…
shumphrey@goktl.com | 919.818.7858

Rachel Thomashow, Consultant
Rachel is an FSQA professional with nearly five years of experience working in the food industry. Her background includes compliance with GFSI requirements and GMPs, supplier documentation, research and development, and product quality verification. She further has experience working in a laboratory and performing analytical tests on food products. Rachel is a PCQI and HACCP certified. She is based in Chicago, IL. Read her full bio…
rthomashow@goktl.com | 847.769.5760
Comments: No Comments
Climate-Related Disclosures: SEC Final Rule
Agencies across the globe have implemented various voluntary and mandatory sustainability and carbon disclosure reporting requirements, ranging from the International Financial Reporting Standards (IFRS) Sustainability Disclosure Standards, to the EU Corporate Sustainability Reporting Directive, to the State of California’s climate disclosure legislation. Even the International Organization for Standardization (ISO) recently issued an amendment to ensure climate change impacts are considered by all organizations in their management system design and implementation.
On March 6, 2024, the U.S. Securities and Exchange Commission (SEC) followed suit, approving its much-anticipated final rule: The Enhancement and Standardization of Climate-Related Disclosures for Investors (Climate-Related Disclosure Rule). Initially proposed in March 2022, the SEC’s final rule requires publicly listed companies to report on greenhouse gas (GHG) emissions; climate-related risks; and other relevant environmental, sustainability, and governance (ESG) information. According to the SEC, the final rules are a continuation of the SEC’s efforts to respond to investor needs for more consistent, comparable, and reliable information about the financial effects of climate-related risks on a registrant’s business.
Overview of Requirements
The SEC’s recent action represents a significant milestone in the U.S., as it integrates climate-related risks and opportunities into business practices and investment decisions. Fundamentally, the final rule requires companies to:
- Identify any material climate-related risks, assess their impacts on financial performance and business strategy, and outline activities to mitigate or adapt to such risks.
- Disclose how they manage the oversight and governance of identified climate-related risk (i.e., the role of management and the Board of Directors).
- Provide information on any climate-related targets or goals that are material to the registrant’s business or financial condition.
- Provide comprehensive disclosure and attestation reporting on Scope 1 (i.e., direct) GHG emissions and Scope 2 (i.e., indirect) GHG emissions (large accelerated filers (LAF) and accelerated filers (AFs)).
- Disclose financial impacts of severe weather events and other natural conditions.
The final rule modifies the March 2022 proposed rule in several ways, most notably by removing the requirement for Scope 3 GHG emission disclosures and providing more time to implement disclosures and related assurance requirements. The SEC Fact Sheet provides a more comprehensive overview of all requirements included in the final rule.
The SEC Fact Sheet provides a more comprehensive overview of all requirements included in the final rule.
Timeline for Compliance
The final rule becomes effective 60 days after publication in the Federal Register. The requirements will be phased in for all registrants with the compliance date dependent upon the status of the registrant as an LAF, AF, or non-accelerated filer (NAF); smaller reporting company (SRC); or emerging growth company (EGC) and the content of the disclosure. Initial deadlines for Scope 1 and 2 GHG disclosures for LAFs begin as early as FY 2026. Refer to the SEC Fact Sheet for a breakdown on all deadlines.
What to Do
If it isn’t happening already, facilities need to start gathering emissions data, develop and implement a system to track GHG emissions, and create an action plan for climate disclosure. Even non-publicly traded companies can benefit from adhering to the general requirements of the new regulations, as publicly traded customers may require climate-related information. This presents an opportunity to demonstrate a commitment to ESG and improve brand reputation; an energy consumption analysis can also lead to improved efficiencies and cost savings.
For many, the SEC’s final rules have the potential to significantly expand requirements beyond what the company is already doing. The transition timeline is designed to allow companies the time needed to:
- Ensure the company has a robust management system in place to provide a solid framework for conducting the required risk assessments, managing new reporting requirements, and organizing required documentation. Note that ISO has taken recent steps to build climate change considerations into all its management system standards.
- Understand current disclosure reporting capabilities, data requirements, processes, and controls, then analyze what climate-related data has already been gathered and what additional information is needed to comply with the final rule.
- Establish or refine corporate governance and define clear roles, responsibilities, and charters for management oversight.
- Develop an action plan for implementation and integrate it with any other existing climate-related initiatives.
As organizations prepare to meet SEC disclosure requirements, KTL can help ensure they have the systems and processes in place to comply and to realize sustainable business value, including:
- Developing and implementing management systems that incorporate climate change considerations, including identifying environmental aspects and impacts, risks, and opportunities.
- Conducting energy audits and analyzing enterprise-wide energy consumption, trends, and opportunities for improvement.
- Calculating and tracking Scope 1, 2, and 3 GHG emissions.
- Identifying and planning for GHG emission reduction targets, renewable energy projects, and other opportunities to improve the organization’s carbon footprint.
- Developing information management tools to manage and track data and trend goals and objectives related to climate change.

Don’t Miss KTL at the 2024 Food Safety Summit
As one of the premier events in the food industry, the Food Safety Summit provides a comprehensive conference and expo for attendees to learn from subject matter experts, exchange ideas, and find solutions to current industry challenges.
- When: May 6-9, 2024
- Where: Donald Stephens Convention Center, Rosemont, Illinois
- Who: Retailers, food processors, distributors, food manufacturers, growers, foodservice, testing laboratories, importing/exporting, law firms, and other food safety professionals
- Find KTL: Stop by our booth (#406) in the exhibit hall!
Tech Tent Case Study Presentation
Be sure to also update your agenda to attend KTL’s Tech Tent presentation on Thursday, May 9 at 11:15 am CT:
Case Study: Leveraging Existing Microsoft® Software to Develop a Robust FSMS
KTL will present a case study demonstrating how Italian Frozen Foods (IFF) USA is leveraging the company’s existing software—Microsoft Power Platform with SharePoint®—to elevate its food safety management system (FSMS) and effectively manage food safety compliance documentation, data, and certification requirements.
- Learn how you can use the software you already have to develop a robust FSMS.
- Understand the approach and process required to ensure successful development and implementation.
- Observe in practice how an integrated system can help organize, control, analyze, and visualize information so you remain in compliance and operate more efficiently.

FAQs on ISO’s New Climate Change Amendments
Effective February 23, 2024, the International Organization for Standardization (ISO) is integrating climate change considerations into all management system standards through its Climate Change Amendments. These Amendments ensure climate change impacts are considered by all organizations in their management system design and implementation.
ISO’s recent action supports the London Declaration on Climate Change of September 2021, which establishes ISO’s commitment to combatting climate change through its standards and publications. The aim of the recent Amendments is to make climate change an integral part of management systems design and implementation to help guide organizational strategy and policy.
What are the changes?
The Climate Change Amendments explicitly require climate change considerations in all existing and future ISO management systems standards, as incorporated into the Harmonized Structure (Appendix 2 of the Annex SL in the ISO/IEC Directives Part 1 Consolidated ISO Supplement). More specifically, the Amendments add the following two new statements to Annex SL for organizations to consider the effects of climate change on the management system’s ability to achieve its intended results:
- Clause 4.1: The organization shall determine whether climate change is a relevant issue, as it relates to understanding the organization and its context.
- Clause 4.2: NOTE: Relevant interested parties can have requirements related to climate change, pertaining to understanding the needs and expectations of interested parties.
The broad scope of these Amendments (i.e., impacting all standards) reflects ISO’s commitment to integrating climate considerations across diverse operational areas (e.g., environment, quality, safety, food safety, security, business continuity, etc.).
What do these changes require?
The original intent and requirements of Clauses 4.1 and 4.2 remain unchanged; however, the Amendments now require organizations to consider the relevance of climate change risks and impacts on the management system(s).
Potential climate change issues will likely differ for the various standards. The Amendments ensure these various risks are considered for each standard and, if actions are required, allow the organization to effectively plan for them in the management system.
What do certified organizations need to do?
Organizations that are certified—or are planning for certification—need to make sure they consider climate change aspects and risks in the development, maintenance, and effectiveness of their management system(s).
The Amendments specifically require these organizations to evaluate and determine whether climate change is a relevant issue within their management system(s). If the answer is yes, the organization then must consider climate change in a risk evaluation within the scope of their management systems. Where relevant, organizations are further encouraged to integrate climate change into their strategic objectives and risk mitigation efforts. The Amendments do not require organizations to do anything about climate change beyond considering the impacts on the management system’s ability to achieve its intended results.
What is the timeline?
The Amendments are effective as of the date of publication. There is no transition for implementation.
Certification bodies and auditors will cover the Amendments in audit activities when assessing this section of a management system. The audit will ensure climate change is considered and, if determined to be a relevant issue, included in company objectives and risk mitigation efforts. If climate change is deemed not relevant, the audit will assess the organization’s process for making this determination.
Will new certifications be issued?
Because the changes are considered a clarification, ISO issued them in the form of an amendment. New standards will not be republished until new versions are released; therefore, the publication year of each ISO standard will not change, and no new certifications will be issued.
What are the benefits of these changes?
The Climate Change Amendments underscore the importance of understanding and addressing the impacts of climate change. By publishing the Amendments in Annex SL, ISO is leveraging the widespread adoption of all ISO management system standards across operational areas to integrate environmental stewardship into organizational practices, promote sustainability, and drive climate change action on a global scale.
For certified organizations, the Amendments are intended to enhance organizational resilience and adaptability to climate-related risks. Considering climate change in this way can significantly contribute to business sustainability and long-term success by:
- Ensuring regulatory compliance (e.g., emission limits, sustainability reporting, etc.).
- Creating positive brand reputation as a sustainable company and associated customer loyalty.
- Managing risks and opportunities associated with supply chain disruptions, energy efficiency initiatives, employee health and safety, natural disasters, etc.
- Engaging employees and attracting new talent who prioritizes sustainability.
- Providing access to markets and investors that have sustainability requirements.

Comments: No Comments
Focus on Employee Safety in the Food Industry
Safety hazards exist in every manufacturing environment. The food industry is no exception. Between October 2018 and September 2019, the Occupational Safety and Health Administration (OSHA) issued a total of 1,168 citations resulting in over $7 million in fines to the food manufacturing industry alone.
Occupational safety and health risks in food manufacturing are often heightened because of the nature of the product (i.e., food or drink) being manufactured. Even food safety measures taken to prevent contamination and ensure food safety can carry inherent occupational safety and health risks.
While food safety is paramount for any company operating in the food industry, a company cannot stay in business if they do not take the appropriate measures to keep employees healthy and safe.
Common Safety Hazards in the Food Industry
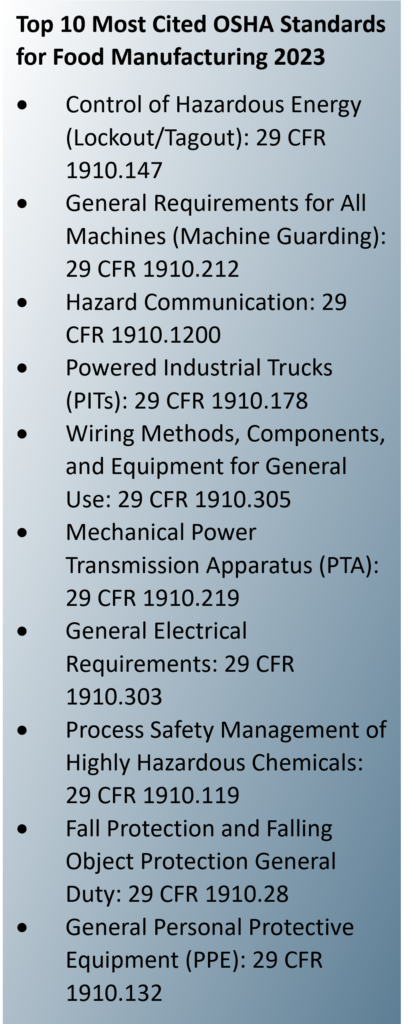
Lockout/tagout and machine guarding recurringly top OSHA’s annual list of the most frequently cited standards in food manufacturing. In fact, the most expensive OSHA fines of 2023 involved two food manufacturers and violations concerning machine guarding and lockout/tagout.
Lockout/Tagout
Employees need to be protected against the unexpected startup or release of energy. Lockout/tagout involves properly de-energizing and securing equipment so it cannot be operated unsafely when a machine needs service or repair. When employees work in a fast-paced environment, they may not take the required steps to first properly de-energize the equipment. As a result, approximately 1,000 workers die each year due to unexpected operation of equipment and/or release of stored energy. Machines and electrical equipment must be properly shut down, de-energized, and locked when servicing.
Machine Guarding
Moving machine parts have the potential to cause severe workplace injuries, such as crushed fingers or hands, amputations, burns, blindness—and most food processing machinery includes pinch points (i.e., blades, rolling parts, presses, etc.) that can put workers at increased risk. Safeguards for machine parts, functions, and processes are essential for controlling hazards and protecting workers from these otherwise preventable injuries.
Other Common Safety Hazards
- Ergonomics. Many food manufacturing jobs involve repetitive motion that can cause musculoskeletal disorders.
- Slips, trips, and falls. Sticky or wet products and frequent cleaning can both contribute to slippery work surfaces that increase the risk for slips, trips, and falls. The high volume of liquids used in food manufacturing and processing creates regular employee exposure to wet surfaces.
- Chemicals and harmful substances. Food facilities rely on various chemical sanitizers and disinfectants to prevent contamination. In addition, anhydrous ammonia, a common refrigerant used in food facilities, is very hazardous (i.e., corrosive, flammable, and explosive), even in small spaces.
- Cut hazards. Knives and other blades are common equipment in food processing plants. Dull blades cause more accidents because they are harder to work with, require more pressure, and may slip more easily. Blades should be sharpened, and employees should wear appropriate PPE.
OSHA Response: Local Emphasis Programs
Between 2016 and 2020, OSHA investigated fatalities, amputations, fractures, and crushed hands and fingers at food manufacturing facilities and identified the primary causes as failure to control hazardous energy or implement adequate machine guarding. In Wisconsin, Bureau of Labor and Statistics (BLS) data from 2011-2020 show that food manufacturing injury rates were consistently higher than the averages for all Wisconsin manufacturing companies. In 2019, OSHA found that food production workers in Ohio had a nearly 57% higher rate of amputations and 16% higher rate of fractures; in Illinois, these rates were 29% higher for amputations and 14% higher for fractures compared to private sector manufacturing.
In 2022, OSHA Region 5 established two Local Emphasis Programs (LEPs)—one for Wisconsin and one for Illinois and Ohio— to encourage employers to identify, reduce, and eliminate exposure to machine hazards during production activities and off-shift sanitation, service, and maintenance tasks (i.e., machine guarding and hazardous energy control – lockout/tagout). These LEPs run through at least 2027 and focus on reducing fatalities and injuries through outreach, education, training, and enforcement activities.
The LEP empowers OSHA to schedule and inspect food industry employers whose injury rates exceed the state average among all manufacturers. The scope of inspections conducted under the LEP focus on reviewing:
- Production operations, sanitation processes, and working conditions.
- Injury and illness records (i.e., OSHA 300 logs), particularly injuries pointing to deficiencies in machine guarding or the hazardous energy control program.
- Machine guarding hazards associated with points of operation, ingoing nip points, and moving or rotating parts of food processing equipment.
- Deficiencies in the hazardous energy control program associated with equipment service, maintenance, setup, and sanitation.
- Hazards associated with chemical burns from corrosives, such as those used for cleaning and sanitizing.
Prepare Your Facility…Protect Your Workers
If you fall under an OSHA LEP, you need to prepare your facility. Even if you don’t, you need to protect your workers.
- Prepare and maintain your OSHA 300 log with any work-related injuries and illnesses. KTL’s OSHA 300 PowerApp can make it easier to collect, search, analyze, store, and aggregate data so it is available when needed.
- Conduct a thorough hazard analysis of the facility, operations, and processes to identify potential safety hazards. The hazard analysis should answer what could potentially go wrong, what the associated consequences are, and how they can be prevented or eliminated.
- Develop, implement, and maintain the appropriate safety programs, procedures, and instructions. A safety management system that aligns with current food safety systems can provide resources to help companies identify and manage safety risks and an organizing framework for policies, procedures, and practices, including:
- Engineering controls for dangerous equipment, including machine guarding.
- Written energy control program and lockout/tagout procedures specific to each piece of equipment.
- Emergency response programs.
- Proper maintenance, cleaning, and sanitation procedures and schedules.
- Guidelines for proper use, care, and replacement of PPE.
- Train your staff. Workers need to have appropriate training, including use of PPE, hazards of extreme temperatures, material handling, hazard communication, lockout/tagout procedures, machine guarding procedures, etc. Initial safety training should be conducted when onboarding new employees, with refresher training provided annually to ensure competency. Training tracking systems allow for the centralized implementation, management, tracking, scheduling, assignment, and analysis of organizational training efforts.
- Provide proper PPE. Workers should be wearing proper footwear, gloves, safety glasses, ear plugs, aprons, etc. and be provided with anti-slip mats to ensure safety.
- Ensure safety data sheets (SDS) are current and available to help identify hazardous chemicals and protect employees from exposure. Train workers on the chemicals used in the workplace, including first aid, what to do in the event of a release, identifying characteristics, proper procedures for working around or with the chemical, and appropriate PPE.
- Use visual communication (e.g., labels, signage) to help protect workers from hot surfaces, exposed moving parts, pressurized systems, hazardous chemicals, slippery surfaces, and more.
Finally, prioritize safety. Small steps can go a long way in making sure employees leave work safely versus becoming an OSHA statistic.
