
Managing Projects & Permits
How many permits does your operating system (e.g., facilities, production, storage, transportation, distribution) have? What kinds of requirements are associated with each of those permits? Who is responsible for making sure requirements are fulfilled? Are there key/critical dates? How many contractors/vendors do you have carrying out activities pertaining to the many diverse permit requirements? How do you manage all that information? And, importantly, how do you verify compliance?
Depending on the breadth and locations of your operations, managing permits and their associated requirements and due dates without a centralized system in place can be an insurmountable challenge. This was certainly true for a large transportation company managing over 3,600 permits for over 1,600 projects across more than 20 states. Finding a better way to track and manage permits wasn’t just a matter of convenience, it was a necessity.
Web-based Tracking System
After a series of washout incidents, the company’s Engineering Department stepped up its efforts to develop a program to ensure engineering and maintenance activities were meeting applicable construction and environmental permit requirements. With so many activities, responsible parties, and deadlines, the Department retained Kestrel Tellevate LLC (KTL) to develop a web-based project tracking system to help:
- Track permit requirements and construction restriction timeframes
- Produce project-specific All Permits Issued (API) documents
- Track post-activity mitigation requirements
- Manage change information
- Report actual-to-budget performance
While the Engineering Department remains responsible for the permitting activities associated with all construction, maintenance, and emergency response activities, KTL’s Permit Tracking System (PTS) offers a cloud-based project management solution to facilitate permit tracking across a variety of data points.
How It Works
The PTS serves as a communication conduit by providing a standardized approach to project/permit activity tracking, while distributing periodic, tailored reports that allow the Engineering Department to manage project activities, as needed. Integrating with internal databases, PTS provides a means to supplement project data with ongoing contractor/consultant input. This enables comprehensive program oversight on the timeliness of the permitting effort and project details, which, in turn, offers preemptive visibility on issues that may affect project construction and permit compliance.
In short, PTS allows the company to:
- Catalog and track permits in one database
- Document and track project conditions, impacts, construction timeframe restrictions, sensitive resources, etc.
- Send and receive notifications of permits about to expire
- Coordinate and communicate with project contractors
- Establish accountability and a standardized approach for reporting and performance measurement
- Effectively manage project process from permitting through handover to construction
- Monitor financial performance
Business Benefits
The Engineering Department has managed nearly 1,600 projects with more than 3,600 associated permits through PTS. Permits in the system include 404 (most common), 401, Floodplain, NESHAPS, FAA, 402, Air, Coast, Air Emissions, 408 Levee, Coast Guard Bridge, Heritage Tree, Tank, Well, Excavated Materials, NPDES, and others.
With this many projects and permits being managed through a consolidated system, PTS is providing many business benefits, including the following:
- Improved program efficiency, consistency, and coherence by fostering a standardized approach to all permitting data management and input by third-party users
- Customized, automated reporting that allows for enhanced progress monitoring, project accountability, and detailed oversight
- Flexible, cloud-based approach to accommodate a variety of program management aspects into a single tool for real-time, comprehensive visibility
- Sole repository for all project management data to help foster communication and coordination both internally and with contractors/consultants
- Improved permit compliance assurance reliability

Getting to the Root Cause
At the most basic level, a root cause is the fundamental reason—or the highest-level cause—for the occurrence of a problem, incident, or event. The root cause sets in motion the entire cause-and-effect reaction that ultimately leads to the problem. Getting to the root cause of any problem is important not just for resolving the issue at hand, but for identifying underlying issues to ensure that similar problems do not occur in the future. This starts with a process called the root cause analysis (RCA).
What Is the Root Cause Analysis (RCA)?
A root cause can be permanently eliminated through process improvement. RCA is a method of problem-solving used to identify the underlying (i.e., root) cause(s) of a problem/incident. RCA can be used to solve problems and provide preventive actions for:
- Major accidents
- Everyday incidents
- Minor near misses
- Human errors
- Maintenance problems
- Medical mistakes
- Productivity issues
- Manufacturing mistakes
- Environmental releases
- Risk analysis, risk mapping
RCA is a systematic process based on the basic idea that effective management requires more than merely putting out fires. RCA focuses on finding a way to prevent these fires from recurring. Rather than just treating symptoms, RCA seeks to identify and address the true, underlying concerns that contribute to a problem or event.
Why is this important? If you just treat the symptoms of the problem, that alleviates them for the short term, but it does nothing to prevent the problem from coming back again. Lasting solutions address the underlying factors—the root cause(s)— that create the problem in the first place. Targeting corrective measures at the identified root causes, subsequently, is the best way to alleviate risk and ensure that similar problems do not occur in the future.
Best Practice
Both the Occupational Safety and Health Administration (OSHA) and Environmental Protection Agency (EPA) encourage organizations to conduct RCA following an incident or near miss at a facility. In fact, facilities covered by OSHA’s Process Safety Management (PSM) standard are required to investigate incidents that resulted in (or could have reasonably resulted in) a catastrophic release of highly hazardous chemicals. Similarly, EPA’s Risk Management Program (RMP) regulations require regulated facilities to conduct incident investigations. In addition, certain management systems, including ISO and Responsible Distribution (National Association of Chemical Distributors) to name just a few, also require RCA.
Whether an organization is subject to PSM, RMP, or management system standards, identifying the root cause of any incident or problem through RCA is a best practice that can significantly benefit organizations by identifying underlying issues to ensure that similar problems do not occur in the future. So, how do you effectively implement RCA?
Six-Step Process
RCA can be broken down into a simple six-step process, as outlined below.
Step 1: Identify and Clearly Describe the Problem
The first step is to understand and document the problem/issue/incident that actually occurred. This might involve interviewing key staff, reviewing security footage, investigating the site, etc. to get an accurate account of the issue. Certainly safety- or security-related incidents might require an immediate fix or prompt action before the carrying out the complete RCA. This is always the first priority.
Some problems are easier to define than others based on what happened and the extent of the issue. When defining and describing the problem, it is important to be as descriptive as possible, as this will aid in future steps to identify the root cause(s).
For example, the first description below is somewhat vague. The second description provides an additional level of detail that more fully documents the situation:
- A forklift driver wasn’t wearing his seatbelt. (vague)
- During a walkthrough of the warehouse on 2/1/20, it was observed that forklift driver John Smith, who is a contract employee, was not wearing his seatbelt while operating the forklift. (clear)
Step 2: Identify Possible Causes…Why?
There are several methods for identifying possible root causes. One of the most common is known as the “5 Why Method”. This approach simply involves asking the question “Why” enough times (i.e., five times) until you get past all the symptoms of a problem and down to the underlying root cause of the issue. The detailed problem description put together during Step 1 serves as the starting point for asking “Why”.
Let’s take our problem description from above a step further to identify the possible causes using the 5 Why Method.
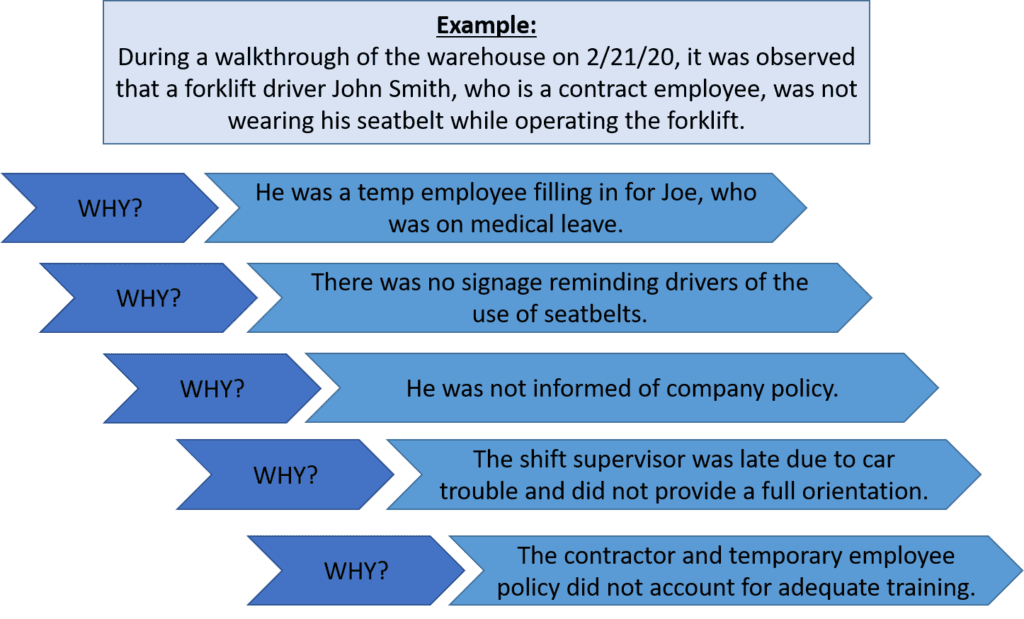 Step 3: Identify Root Cause(s)
Step 3: Identify Root Cause(s)
At this point, the 5 Why Method is leading you to the core issue that set in motion the entire cause-and-effect reaction and, ultimately, that led to the identified problem(s). It’s now time to determine whether the five whys have dug deep enough. Where does your questioning lead you? Is there one root cause or are there a series of root causes contributing to this incident? Often, there are multiple root causes that may be factors to address when preventing future incidents.
In our forklift operator case, the 5 Why Method points to the lack of a standardized checklist of all items to be trained on—including forklift training—prior to a new contract employee coming onsite.
Step 4: Corrective and/or Preventive Action Taken
Based on the identified root causes, it then becomes possible for the facility to determine what corrective and/or prevention actions (CAPAs) can be taken to fix the problem and, just as important, prevent it from occurring in the future. For our example, there are a number of potential CAPAs:
- Stop the employee from operating the forklift and educate him on seatbelt policy prior to resuming work
- Review contract/temp employee training program
- Retrain shift managers on training expectations
- Obtain training records for contract/temp employees
- Provide refresher/retraining, as necessary
- Add signage to forklifts and warehouse bulletin boards about seatbelt policy
Step 5: Analyze Effectiveness
The effectiveness of whatever action is taken in step 4 needs to be evaluated to determine whether it will resolve the root cause. If not, another CAPA should be explored, implemented, and analyzed to assess its impact on the issue/problem. If it is a root cause, it should help to resolve the issue and you should move on to step 6 below.
Let’s return to our example. You might ask, “Was the retraining effective?” An evaluation shows the following:
- Yes, the employee continues to operate the forklift using seatbelt.
- Yes, subsequent walkthroughs of the warehouse over the next six months have not resulted in any additional seatbelt violations.
- The next contract/temp employee brought on to assist during the busy end-of-year season was required to produce current training.
Step 6: Update Procedures, as necessary
As CAPAs are implemented, once they prove effective, related policies and procedures must be updated to reflect any changes made. This step ensures the outcomes of the RCA will be integrated into operations and used to prevent similar incidents from happening in the future.
In our current example, this might mean that the Contractor Policy is updated to include a new section specific to the hiring of contract/temp employees with the following requirements:
- Obtain valid training certificates for work performed
- Ensure Managers conduct on-the-job training for contract/temp employees specific to work performed
Benefits of RCA
Following these six steps will help to ensure a thorough investigation that identifies the root cause(s) versus just symptoms is conducted. It further ensures that any changes related to the root cause are integrated into the organization to prevent similar events from happening again. In the end, the RCA process can help:
- Reduce the risk of injury and/or death to workers and community members
- Reduce the potential for environmental damage
- Avoid unnecessary costs resulting from business interruption; emergency response and cleanup; increased regulation, audits, and inspections; and OSHA or EPA fines
- Improve public trust by maintaining an incident-free record
- More effectively control hazards, improve process reliability, increase revenues, decrease production costs, lower maintenance costs, and lower insurance premiums

Employees Need Rules, Not Regulations
KTL recently announced our partnership with Martin Mantz Compliance Solutions (Martin Mantz), developer of the GEORG Compliance Management System® software. KTL is providing regulatory compliance expertise to the German-based company as it expands its offerings to clients with operations in the United States.
In this recent article, our partners at Martin Mantz discuss how Rudolph Logistics Group, an international logistics service provider from Germany, is using GEORG as a compliance solution to provide employees clear information in accordance with ISO standards on:
- Tasks – what they have to do
- Responsibilities for implementation – who needs to do it
- Date/time of completion – when it needs to be done
- Description of the way the task is to be performed – how the task must be fulfilled
The objective is to simplify requirements to the extent possible so employees can focus on tasks to be completed without needing to interpret complicated and extensive guidelines. Read more…

April 16 Webinar: Improving EHS Management with IT
Effective information management is critical to complying with complex EHS regulations. Join KTL and Southeast Missouri State University (SEMO) for this APPA webinar to get helpful tips for the successful management of EHS information, data, documents, and records.
Improving EHS Management with Information Technology: A University Demonstration
April 16, 2020 | Noon – 1:00 p.m. CT
REGISTER NOW!
This webinar will use a formal EHS management system model (plan-do-check-act) to highlight the importance of:
- Identifying, understanding, and documenting applicable EHS requirements
- Providing easy-to-use EHS information management tools
- Capturing institutional knowledge of experienced staff for operational sustainability
Learning Objectives
Facility managers, plant operators, EHS staff, and supervisors working in higher education will better understand:
- Key components of an effective EHS management system based on ISO 14001/45001
- Best practices for applying information technology to assist with EHS compliance
- Strategies for improving adoption of new technology tools throughout campus
- How to use an affordable, available technology platform (Microsoft SharePoint®) to enhance EHS compliance and communication practices
Presenters
SEMO’s Autumn Gentry will join KTL Principal Joseph Tell to provide a demonstration of SEMO’s recent efforts to manage and communicate EHS information using Microsoft SharePoint® tools to simplify EHS compliance.
About APPA, Leadership in Educational Facilities
Formerly known as the Association of Physical Plant Administrators, APPA is recognized globally as a leader in professional development programs, credentialing, research, publications, networking, and information services for the educational facilities profession. APPA’s mission is to “support educational excellence with quality leadership and professional management through education, research and recognition.”

Comments: No Comments
KTL Announces Partnership with German Company Martin Mantz
KTL is pleased to announce our partnership with Martin Mantz Compliance Solutions (Martin Mantz), developer of the GEORG Compliance Management System® software. KTL is providing regulatory compliance expertise to the German-based company as it expands its offerings to clients with operations in the United States.
“Martin Mantz has created something unique with the GEORG software in that it simplifies and provides an interpretation of legal and technical requirements in a customer-specific database,” KTL Principal Lisa Langdon states. “KTL’s understanding of industrial operations, as well as U.S. legal and technical requirements (e.g., EPA, OSHA, FDA, ISO), allows us to translate these requirements into simple tasks in the GEORG system that employees can follow to help fulfill regulatory requirements.”
How GEORG Works
GEORG is used to make the requirements of standards and regulations comprehensible and transparent. KTL specializes in the practical mapping of legal requirements and audits. These audits allow KTL to create technical content for the GEORG system based on facility-specific applicability. We then work directly with the company to delegate the identified tasks. If there are revisions in the standards/regulations, KTL works in the system to ensure tasks are updated to meet regulatory requirements.
The benefits of this approach include:
- Effectiveness – All tasks are assigned, easily formulated, and regularly updated.
- Efficiency – The effort and expertise required to understand complicated regulations is reduced.
- Transparency – Responsibilities are clear and easily visible to all employees.
- Conformity – Compliance status within the system reflects the degree of fulfilment of the related requirements.
Faber-Castell Expands GEORG Implementation to U.S. Subsidiary
Faber-Castell Cosmetics, an internationally renowned Martin Mantz customer with worldwide operations, is already benefitting from the Martin Mantz-KTL partnership. After successful implementation of the GEORG software in their German facilities, Martin Mantz has worked with KTL to expand usage to Faber-Castell’s subsidiary in the U.S.
About Martin Mantz Compliance Solutions
Martin Mantz Compliance Solutions, based in Grosswallstadt and Leipzig, Germany, offers its contractual partners services in the area of legal organization (GEORG) of companies to avoid organizational negligence and compliance violations. This includes consulting and provision of the compliance software GEORG Compliance Management System®, implementation of the technical and legal modules, as well as construction and maintenance of the customer-specific database. https://www.martin-mantz.de/
About Kestrel Tellevate LLC
KTL is a multidisciplinary consulting firm that specializes in providing environmental, health, and safety (EHS) and food safety management and compliance consulting services to private and government clients. Our primary focus is to build strong, long-term client partnerships and provide tailored solutions to address regulatory requirements. KTL’s services include management system development and implementation, auditing and assessments, regulatory compliance assistance, information management solutions, and training. KTL is a Small Business Administration-registered company with headquarters in Madison, WI and Atlanta, GA and offices across the Midwest and Washington, D.C. www.kestreltellevate.com

Comments: No Comments
Managing Aerosol Cans as Universal Waste
Aerosol cans have long provided regulatory challenges under the U.S. Environmental Protection Agency (EPA). Some states have defined aerosol cans as universal waste; some states define it as reactive (D003) waste. On December 9, 2019, the EPA published a new rule (effective February 7, 2020) adding hazardous waste aerosol cans to the Universal Waste Program under the federal Resource Conservation and Recovery Act (RCRA) regulations.
This change provides a clear, protective system for managing discarded aerosol cans. The streamlined universal waste regulations are expected to ease regulatory burdens on retail stores and others that discard hazardous waste aerosol cans; promote the collection and recycling of these cans; and encourage the development of municipal and commercial programs to reduce the quantity of these wastes going to municipal solid waste landfills or combustors.
Current Review of Universal Waste
The designation of universal waste holds several advantages for generators. Universal waste doesn’t “count” against generator status. It does not have to be manifested and generally requires specific labeling language.
Under EPA’s definition, the following are the current universal waste streams:
- Batteries (Li, Ni-Cd, Ag, Hg)
- Mercury-containing equipment (MCE)
- Electric lamps
- Cathode ray tubes (in electronics)
- Pesticides (recalled or farmer-generated)
Adding Aerosol Cans
Adding aerosol cans to the EPA’s Universal Waste Program now provides the option for generators to manage the waste as hazardous or universal. The program addresses emissions with more stringent language and allows generators to set up separate management.
More specifically, the following outlines some basic details of the program for effectively managing aerosol cans as universal waste:
- If the aerosol can is empty (i.e., at ambient pressure, there is no more liquid inside), it is considered an empty container. It can be recycled as metal or thrown away as solid waste (except for Nebraska, where it is D003).
- Full and partial aerosols can be recycled.
- Depending on the vendor, segregation may not be required.
- If the aerosol can is punctured, contents must be captured, and a hazardous waste determination must be performed.
- If the waste from the aerosol can is hazardous, the contents count toward generator accumulation.
- The only benefit to generators occurs if they ship unpunctured cans for recycling.
- If a household hazardous waste (HHW) facility accepts business aerosols and punctures them, the HHW facility becomes a generator.
- All emissions must be captured and managed properly; filters may be hazardous.
Regulatory Review
The new program for managing aerosol cans requires a safety program, education, and written documentation. KTL has the experience and expertise to help you evaluate your waste and to properly manage universal waste. Although not as complex as the requirements for proper hazardous waste management, universal waste has nuances that a generator must be aware of to properly meet the regulatory requirements. KTL can help determine how this new regulation applies and if it can help you minimize your regulatory burden, save some money, and manage your waste more efficiently.

Principles of Auditing
To ensure companies uphold standards (internal or external) and continuously improve performance, audits are critical. In short, there are three primary purposes of auditing:
- Verify conformance with the standard/requirement – Are we doing what the standard/requirement says we must do?
- Verify implementation of stated procedures – Are we following the steps in our documented procedures?
- Evaluate effectiveness – Are we accomplishing our goals and objectives?
For an audit to be effective, appropriate mechanics must be in place when it comes to planning, execution, and reporting.
PLANNING
As with most things, your execution will only be as good as your plan. All good audits must begin with planning. This involves everything from planning for your team, to planning out the scope of the audit, to planning all the associated logistics.
Auditors: Who Is on the Team?
Depending on the size and complexity of the audits, audit teams need to be selected. These individuals must be independent of the area being audited and trained in the basic elements of the facility’s management system and/or programs. Team members will be led by a trained auditor. The auditor’s responsibilities include the following:
- Comply with and communicate audit requirements
- Prepare working documents under the direction of the Lead Auditor
- Plan and carry out the assigned responsibilities within the scope of the audit
- Collect and analyze evidence to draw conclusions
- Document audit observations and findings
- Report audit results to Lead Auditor
- Retain and safeguard audit documents
- Cooperate with and support the Lead Auditor
- Assist in writing the report
As indicated above, one person on the team is typically designated the Lead Auditor. This individual will coordinate audit assignments and address any questions/concerns that may arise. Specifically, the Lead Auditor has the following responsibilities:
- Assigns team members specific management system/program elements, functions, or activities to audit
- Provides instructions on the audit procedure to follow
- Makes changes to work assignments, as necessary, to ensure the achievement of audit objectives
Audit Objectives, Scope, and Plans: What Are We Auditing?
The audit is all about:
- Conformance – auditing sections of the standard/requirements to determine if the system conforms
- Implementation – auditing work instructions to see if they are being followed
In determining the audit scope, it is importation to define what is to be audited (e.g., policy, planning, implementation, checking/corrective action, management review). If the organization has more than one physical location, the scope may outline what physical locations and/or organizational activities are to be audited (e.g., production lines or departments). These factors will ultimately also help determine the length of the audit.
Logistics: How Are We Going to Do This?
There are many things to factor into the audit from a logistical standpoint for it to go smoothly. Safety should always be of utmost concern. What precautions do auditors need to take? Is there any PPE that might be necessary? Do auditors need any special safety training introduction or training before conducting the audit? Consider the facility. Auditors need to understand the operation/activity being audited. In line with this, the auditor must also have an understanding of whether there is any equipment or special resources needed, ranging from technical support (e.g., tablets, smartphones) to lunch. Finally, it is important to make sure there are no conflicts of interest when it comes to the auditor and the facility that is being audited.
EXECUTION
Once planned appropriately, audits should be conducted according to the program elements. Interviews and objectives evidence will both provide the support needed to conduct a valid audit.
Program Elements
The auditor must know in advance which elements are being covered in an audit so he/she can:
- Control the pace of the audit.
- Guide the course of the audit.
That being said, additional audit activities should not be restricted if other issues arise.
Auditing should only be done against current controlled work instructions or procedures related to the program elements. Procedures that are being used in the field must be verified. Historical and/or uncontrolled procedures should not be used.
Auditors must remember that they are creating a record. Notes should include statements, document numbers, identifiers (e.g., department, area), positions. Common pitfalls to be avoided in taking notes include abbreviations, no location identifier for observations, no document references, illegible, pejorative, cryptic. These things all impact the credibility of the audit.
Interviews
The goal of an interview in the audit is to obtain valid information. However, how questions are asked will impact the answer. Auditors must prepare and know what questions need to be asked and how to ask them in advance of the audit. Creating an atmosphere of trust and open communication is key to getting open and honest responses. Remember, the goal is to audit the system, not the interviewee.
The following are good rules of thumb for conducting effective audit interviews:
- Direct questions to the person who does the job, not to the supervisor.
- Never talk down to anyone.
- Speak the person’s language.
- Speak clearly and carefully.
- LISTEN!
- Use who, what, where, when and why in your questioning vs. can or does.
Objective Evidence
Objective evidence provides verifiable information, records, or statements of fact. This is vital in any audit report. Objective evidence can be based on any of the following:
- Interviews
- Examination of documents
- Observation of activities and conditions
- Results of measurements
- Tests
- Other means within the scope of the audit
Evidence should be firsthand evidence based on witnessed fact, not supposition, presumption, hearsay, rumor, or conjecture. It can be qualitative or quantitative, but it should be repeatable.
REPORTING
Findings form the basis of the report. Findings can be classified in one of two ways:
- Nonconformance is the observed absence of or lapse in a required procedure or the total breakdown of a procedure that can cause a negative impact on the organization’s environmental performance. These can fall into a few categories:
- Does not meet the requirements of the standard. This may include issues identified with records, procedures, work instructions, and use of controlled documents.
- Is not fully implemented. Most commonly, these implementation nonconformances may relate to training, communication, and documentation.
- Is improperly implemented. This is often demonstrated by worker lack of understanding, improper implementation of written work instruction, or missing stated required deadlines.
- Opportunity for improvement is just that—an opportunity to improve management to either reduce impacts, minimize legal requirements, prevent future nonconformances, or improve business performance.
The following examples and tips can serve as guidelines for writing useful and more concrete findings that will the company to identify opportunities for improvement:
- Do not overstate conclusions.
- Poor: The procedure for handling spent light bulbs is being ignored.
- Better: Three spent fluorescent bulbs were found in the general trash.
- State the problem clearly and exactly.
- Poor: Instruments are not being calibrated.
- Better: The sampling and analytical instruments in the wastewater treatment plant are not calibrated.
- Avoid generalities.
- Poor: The area’s empty drum management process is inadequate.
- Better: The hi-lo driver in the area handling empty drums was not trained on hazardous material handling.
- Communicate the extent of the problem fully.
- Poor: All cardboard in the catalytic converter area is being sent to the compactor.
- Better: None of the cardboard in the catalytic converter area was being stored and/or evaluated for reuse as dunnage.
- Do not focus on criticisms of individuals.
- Poor: Jim Jones had no understanding of the safety policy.
- Better: Discussions with several employees indicated that the safety policy was not fully understood.
- Give specific references.
- Poor: Hazardous waste area inspections have not been conducted.
- Better: Weekly hazardous waste storage area inspections (VMEWP-008) have not been conducted since June 2002.
- Avoid indirect expressions.
- Poor: There were occasions when the reports were not filed on time. It appears the air monitoring equipment is not calibrated.
- Better: Reports were filed late on ten occasions in 2002. There were no records of air monitoring equipment calibrations for 2001 or 2002.
Audits are a skilled activity. They provide the basis for assessment of conformance and, correspondingly, company actions to improve performance. For audits to be valuable, however, the audit process must be consistent and controlled. Clearly and correctly documented nonconformances lead to appropriate corrective actions—the mechanism for translating audits into improvements.

Comments: No Comments
The Latest on RMP: Reconsideration Rule Finalized
The Environmental Protection Agency’s (EPA) Risk Management Program (RMP) Rule requires facilities storing specific chemicals above certain threshold amounts to develop risk management programs to prevent and mitigate accidents that could release those chemicals into the environment. Just what the RMP rule entails has been the subject of debate since EPA first proposed the RMP Amendments in 2016. Rules related to RMP requirements have been published, petitioned, delayed, vacated, reissued, and reconsidered. As the most recent action in the ongoing RMP saga, EPA Administrator Andrew Wheeler signed the RMP Reconsideration Rule on November 21, 2019.
Reconsideration Rule
According to EPA Administrator Wheeler, the intent of the RMP Reconsideration Rule is to promote “improved coordination between chemical facilities and emergency responders, reduce unnecessary regulatory burdens, and address security risks associated with previous amendments to the RMP rule.”
What does that entail? In the final Reconsideration Rule, many of the major provisions that were added in the RMP Amendments Rule are rescinded, including the following requirements to:
- Hire a third-party to conduct a compliance audit after an RMP reportable accident. EPA retains the right to still require a third-party audit, when appropriate.
- Conduct a Safer Technologies and Alternatives Analysis (STAA). Again, this can still be required by EPA but is already encouraged under the rule’s existing Process Hazard Analysis (PHA) provisions.
- Conduct and document a root cause analysis after an RMP reportable accident/near miss, in efforts to maintain consistency with the OSHA Process Safety Management (PSM) standard.
- Make very broadly defined information available by facility to the public upon request to alleviate potential security/terrorism threats.
A number of other requirements, particularly as it relates to local emergency coordination and training exercises have been retained and/or modified, as follows:
Retained
- Requirements that facilities must coordinate annually and document coordination with local response organizations.
- Annual notification drills to confirm that emergency contact information is accurate.
- Requirement to perform field and tabletop exercises as a way to train facility personnel and local responders.
- Frequency of 3-year tabletop exercises to ensure regular emergency training is conducted.
Modified
- Provision to reduce potential security risks associated with avoiding the open-ended information disclosure provision.
- Frequency of field exercises by removing the ten-year minimum requirement to reduce burden on local emergency responders.
- Scope and documentation provisions for field and tabletop exercises to reduce burden.
- Requirement to hold a public meeting after an incident that has offsite impacts vs. releases with only onsite impacts.
The EPA RMP Website provides additional information and resources, as well as a copy of the complete RMP Reconsideration Rule and the updated requirements.
Risk-Based Approach
According to the EPA RMP Reconsideration Final Rule Fact Sheet, the final rule retains the prevention provisions that have resulted in the trend of fewer significant chemical accidents, which have declined more than 50% since RMP was first published in 1999. With the RMP Reconsideration Rule, EPA’s intent is to take a more risk-based approach that focuses on the highest risk facilities (i.e., the less than 2% of RMP facilities reporting multiple releases), as opposed to the 90+% of RMP facilities who reported no accidents from 2007-2016.
Industry Reaction
RMP regulates approximately 12,500 facilities, including agricultural supply distributors, waste/wastewater treatment facilities, chemical manufacturers and distributors, food and beverage manufacturers, chemical warehouses, oil refineries and other chemical facilities. As a whole, industry has commended EPA for taking a more risk-based approach to RMP that reduces the regulatory burden on industry:
- “The RMP program has been working very well. The data clearly shows a continuous reduction in accidents of regulated facilities. It is important for EPA to focus on compliance assistance efforts and promoting enhanced coordination between RMP facilities and local first responders.” Richard Gupton, Senior Vice President of Public Policy and Counsel, Agricultural Retailers Association (ARA)
- “SOCMA and its members have been actively engaged in the reconsideration of the 2017 RMP Amendments and has been supportive of the Agency’s efforts to delay implementation of the prior rule while it conducted reconsideration proceedings. This final rule will provide much-needed certainty at facilities seeking to understand and achieve their RMP compliance obligations.” Robert Helminiak, Vice President of Legal and Government Relations, Society of Chemical Manufacturers and Affiliates (SOCMA)
- “We commend the EPA for developing a rule that reflects the extensive feedback the agency received through a comprehensive and thoughtful process to seek public input. The agency wisely incorporated the recommendation to strike the right balance of sharing vital safety information with emergency responders and protecting sensitive security information. EPA also followed the recommendation to utilize the EPA’s latest data to identify areas where more focused compliance assistance is needed to help facilities further reduce the number of reportable safety incidents.” Mike, Walls, Vice President of Regulatory and Technical Affairs, American Chemical Council (ACC)
- “NACD commends EPA for taking our recommended changes to the 2017 rule seriously and for instead pursuing a commonsense approach that improves facility safety without hamstringing businesses with burdensome requirements that have no proven benefit.” Jennifer Gibson, Vice President of Regulatory Affairs, National Association of Chemical Distributors (NACD)
Implications/What’s Next?
As the regulatory history with the RMP Rule demonstrates, it remains important for impacted companies to stay on top of the requirements, coordinate efforts with local emergency responders, and plan accordingly. For companies impacted by the RMP Reconsideration Rule, it is important to:
- Understand the hazards posed by chemicals at the facility
- Assess the impacts of a potential release
- Design and maintain a safe facility to prevent accidental releases
- Coordinate with local emergency responders
- Minimize the consequences of accidental releases that do occur
Kestrel Tellevate LLC (KTL) has experience working with a broad cross-section of industries impacted by RMP, particularly chemical companies. We have created RMP and General Duty Clause audit protocol and conducted audits and investigation/improvement programs following significant release events. We also routinely work with Local Emergency Planning Commissions (LEPCs) to coordinate emergency response efforts and exercises.
Our understanding of the regulations and industry needs spans years of experience and commitment to helping industry comply with regulations and operate more efficiently. For example, KTL developed and implemented the Guidance Manual and Training Modules for the Responsible Care Management System (RCMS) and RC14001, ACC’s management system integrating environmental, safety/process safety and security. KTL is also a Preferred Provider of compliance services for NACD member companies and a recognized NACD Responsible Distribution Adviser, providing in-depth support for members and affiliates during Responsible Distribution implementation and integration with other management systems and EHS compliance initiatives.
Regulatory enforcement-driven projects such as those related to RMP require skills in regulatory strategy, negotiations, expert analysis, presentations and testimony—and, equally important—trust and relationship building. KTL can work with companies to:
- Identify/understand/prioritize compliance risks
- Outline steps to improve performance
- Define organizational roles and responsibilities
- Streamline compliance methods
- Plan and conduct required tabletop exercises and coordinate with local emergency response
- Implement, monitor, and continually improve

4 Steps to Reporting Audit Results
The audit report communicates the information, findings and opinions derived from the audit. The report communicates either acceptability of the current status of the management system or reports non-conformances that need corrective action. The following outlines the suggested steps for reporting audit results.
Step 1. Assess the Status of Current Internal Controls
One of the auditor’s main responsibilities is to evaluate whether the current internal controls that govern the management system are adequate. Do the audits:
- Highlight areas of concern or hazards that may be a failure waiting to happen?
- Focus attention of the 20% of the factors that cause 80% of the problems?
- Help to eliminate ineffective controls or make existing controls better?
- Aid in the detection and prevention of deficiencies or non-conformances?
- Look through and investigate possible “homeblindness”?
- Verify the management system links are supportive and feed each other information to assure continual improvement?
The auditors must constantly challenge the status quo and push the management system forward beyond its comfort level.
Step 2. Prepare Audit Report
Most facilities use a formal audit report system. The audit report format is prescribed and followed by the auditor. The auditor typically states:
- Date and time of audit
- Department audited
- Management system clauses audited to
- Personnel interviewed
- Documents reviewed
- Summary of findings
- Conformance or non-conformance determination
Step 3. Discuss Audit Findings
The lead auditor will then take the completed audit report and review the contents with the affected department head. Upon acceptance by the department head, the final audit report should then be signed by the department head verifying acceptance and responsibility for any change(s) required.
Step 4. Determine Plan of Action
The entire reason for conducting internal management system audits is to verify conformance and continually improve on the management system. Therefore, it is extremely important that all identified non-conformances are corrected in a timely manner.
Some companies place all audited non-conformances into their corrective/preventive action process for tracking purposes. Others place only critical non-conformances into the corrective/preventive action process. Regardless of the mechanics of tracking the identified audited non-conformances, it is imperative that corrective action is taken.
Once the corrective action is in place, the auditors should review the actions taken and verify the root cause was identified properly and resolved. An accept or reject decision can then be rendered for the change action.
If acceptable, no further action is required, and the issue is considered resolved. If unacceptable, the department head must complete a new root cause analysis, develop a new action plan, and put the new action plan into place. The auditors will now review the new action plan and make a determination of acceptance or rejection.
Audit Team Members
It is advisable to rotate your internal management system audit team members. This will allow for fresh perspective and a new set of eyes to look at the management system. This serves many purposes:
- Gives a specific timeframe of responsibly for those thinking of enlisting as an auditor
- Allows for gradual increase of responsibility over time; new auditors learn and perform audits, older auditors become mentors for the new auditors, graduates leave program and are viewed by company personnel as “knowledge experts” on the management system
- Allows for fresh perspective on auditing
- Trains numerous employees on the management system
- Reinforces the concept of continuous improvement
Are You Prepared?
Use your answers to the questions below to evaluate your preparation for reporting audit results.
- Has the auditor evaluated the current internal controls for suitability, adequacy, and effectiveness?
- Does the auditor have hard copy evidence of conformance and/or non-conformance?
- Have all questions prepared prior to the audit been satisfactorily answered and explained?
- Is the audit report clear, concise, and informative?
- Does the department head agree or disagree with the findings?
- Are all identified non-conformances tracked and resolved in a timely manner?
- Based on audit non-conformances, are procedures and work instructions being changed and improved?
- Do employees understand the management system is being audited, not the employee?
- Is change readily accepted by company personnel?

Comments: No Comments
Top 6 Best Practices for Waste Management
BIOTECHNOLOGY FOCUS
Identifying and managing your wastes is not a task to be taken lightly. If waste is incorrectly managed, there are regulatory compliance risks, exposure risks, and potential financial penalties that can impact your business. Given these risks, effective waste management requires investments of time, money, and resources. However, if you are proactive in your efforts to inventory your wastes, understand your requirements, and develop a plan to manage your inputs and outputs, it is possible to turn those investments into value for your organization.
The following six best practices can help ensure you are you managing your waste correctly, efficiently, and cost-effectively—and that you can sustain those efforts for the long term:
1. Inventory your wastes – Biotech labs and industrial processes traditionally produce many different types of waste that can present significant waste management challenges. A methodical, analytical approach to characterizing and evaluating waste can substantially improve efficiencies when it comes to handling waste and minimize the risks of improper waste management. This is done through an EPA-required waste determination. In addition to reviewing chemicals that are used in processes and the different types of risk they present, a waste determination should evaluate allwaste being generated by processes throughout the facility.
2. Understand your compliance requirements – The Environmental Protection Agency (EPA) regulates much of the waste generated by industry. Over the past ten years, the Agency has demonstrated an even stronger focus on labs. Additional regulatory agencies that oversee lab and industry operations include the Occupational Safety & Health Administration (OSHA), Department of Transportation (DOT), Department of Homeland Security (DHS), fire department, and others depending upon the type of work being done, chemicals being used, and resulting end products. The waste scenarios seen in labs and industry are countless, and each may hold associated regulatory compliance requirements. While this clearly presents business risks, it also provides a unique opportunity to create strategies to manage wastes more effectively and efficiently, improve safety, and reduce the potential costs of regulatory compliance.
3. Understand your business processes – Companies who want to proactively manage their waste need to go through the process of understanding where your waste fits into your business processes—and what you need to do with not only your waste, but also your operations, to minimize risk, reduce costs, and ensure compliance. Having business and production processes mapped out helps companies improve the interconnected set of processes, sub-processes, activities, and tasks that allow the business to manage waste most effectively. A thorough review of business and operational processes and the waste being generated further creates the opportunity for a “bottom-to-top” evaluation of all regulatory compliance. It is a process of understanding what you have, where it fits, and what you need to do with it to minimize risk, reduce costs, and ensure compliance.
4. Get the right parties trained – One of the most common violations identified by both DOT and EPA is failure of personnel signing hazardous waste manifests to have appropriate DOT hazardous waste training. Failure to meet this training requirement can result in substantial financial penalty. Perhaps even more important, lack of training may also impact the understanding of employees in how to correctly—and safely—perform their duties. There are many people who touch waste at various points in the process and they all need to be trained on how to work with it in a manner that is safe and compliant with regulatory requirements.
5. Develop waste management strategies – Are there waste streams that you are paying too much to manage? Are there alternatives to the reagents or kits you are using that may minimize your risk and improve safety in your lab? Are there strategies that can make waste management simpler, more cost-effective, and more compliant that you could implement in your lab? As key indicators of waste quantities are identified, strategies for internal process changes that can minimize waste generation can be implemented.
When developing your strategies, focus on managing waste as close to the source(s) as possible. There are frequently alternatives to hazardous, universal, biohazardous, and special waste management that will minimize risk and improve financials. These waste minimization strategies need to be identified and evaluated to determine their applicability and potential impacts. Options that Kestrel has investigated and assisted with implementing include:
- Treating hazardous waste to minimize quantities for disposal
- Recycling used solvents through evaporation and reclamation strategies
- Solidifying nonhazardous waste waters with associated subtitle D landfilling
6. Establish a system to sustain ongoing compliance – Documenting waste management procedures and processes, along with management oversight and continual review and improvement, is key to ensuring ongoing compliance. Technology (i.e., a compliance information management system (IMS), apps, tools) can help create process standardization, operational efficiencies, and, subsequently, consistent and reliable compliance/waste management performance. Do you have permitting requirements? Does your staff need training? How do you maintain your records? Are there regular (e.g., annual, semi-annual) plans and/or reports you need to submit? Do you have routine inspections and monitoring? All these things can and should be built into a compliance IMS so they can be managed more efficiently.
Through an evaluation of chemicals onsite, development of an inventory of both chemicals used and waste generated, and identification of processes to efficiently and effectively manage waste, businesses/labs can ensure they understand and meet their EHS regulatory obligations in the most efficient ways possible.
