
Now Hiring: Safety Consultant
Location: Chicago, Illinois or St. Lous, Missouri
KTL is seeking an experienced Safety Consultant based in the Chicago, IL or St. Louis, MO metropolitan area with a strong safety compliance background working as a consultant or in industry to join our team. This individual will have knowledge of general industry safety procedures and Occupational Safety and Health (OSHA) requirements. The Safety Consultant will have experience auditing, developing, and implementing OSHA safety programs in an industrial setting, and expertise helping organizations proactively manage their operational safety-related risks.
Responsibilities and tasks include the following:
- Conducting OSHA compliance audits in general industrial settings.
- Researching federal, state, and local regulatory requirements and helping maintain standards.
- Effectively managing and building client relationships, leading to repeat business.
- Developing and delivering training and coaching on safety.
- Performing site safety assessments, conducting incident investigation/root cause analysis, and implementing follow-up corrective actions.
- Working with a team to assess, design, implement, and audit safety management systems and related programs.
- Designing safety performance metrics to drive continual improvement.
- Applying quality and process improvement methods and tools.
- Writing technical reports, work plans, and proposals.
- Supporting other KTL professionals to effectively manage and deliver projects.
- Managing tasks remotely and at client sites.
Requirements
- B.S. degree in Environmental Science, Engineering, or Occupational Safety preferred.
- 5-10 years of consulting or relevant experience working in safety for industry/manufacturing.
- Associate Safety Professional (ASP) or Certified Safety Professional (CSP) preferred.
- Recent experience conducting safety audits.
- Strong knowledge of OSHA safety standards and programs.
- Experience with environmental compliance preferred.
- Experience in healthcare preferred.
- Previous project management experience and ability to manage multiple projects and clients.
- Excellent verbal and written communication, interpersonal, and presentation skills.
- Excellent research, analytical, writing, and organizational skills.
- Ability to work independently and as a part of a team.
- Proficient in Microsoft 365 software.
- Valid driver’s license.
- Ability to travel up to 50%.
How to Apply
Forward a resume to recruiting@goktl.com.
Company Description
KTL is a multidisciplinary consulting firm that specializes in providing environmental, health, and safety (EHS); food safety; and quality management and compliance consulting services to industry and government clients. Our primary focus is to build strong, long-term client partnerships and provide tailored solutions to address regulatory compliance requirements. KTL’s services include auditing and assessments, management system development and implementation, certification support, regulatory compliance assistance, information management solutions, and training. Our headquarters are in Madison, WI and Atlanta, GA, with satellite offices throughout the U.S.

Don’t Miss KTL at the 2024 Food Safety Consortium
KTL is excited to be joining the 2024 Food Safety Consortium in Washington, DC, October 20-24, 2024. The 13th Annual Food Safety Consortium provides food safety and quality assurance professionals with cutting-edge knowledge, practical skills, and a collaborative network to enhance their professional development as champions of food safety.
KTL will be leading the following breakout session as part of the workshop’s technical agenda:
The Big Secret…You already have the software you need to manage food safety
October 22, 2024 | 8:30-9:15 am | Presenter: Roberto Bellavia, Senior Consultant and Partner
This session will demonstrate how food companies are leveraging Microsoft Power Platform with SharePoint® to elevate the food safety management systems (FSMS) and more effectively manage food safety compliance documentation, data, and certification requirements.
And be sure to stop by and visit us at Booth #11. We look forward to seeing you at the Food Safety Consortium!

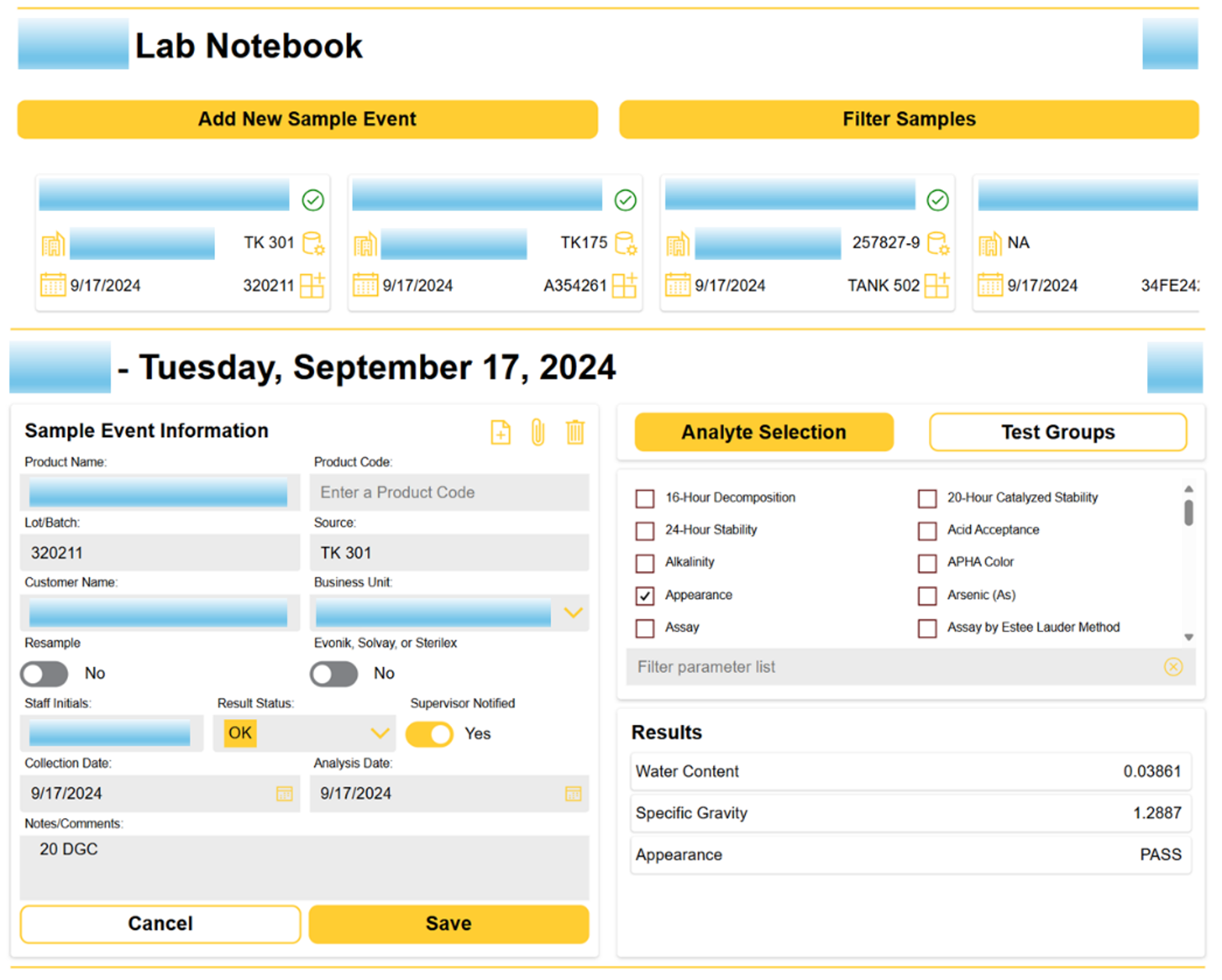
Tech Corner: Laboratory Information Management System
Functionality: What does it do?
Laboratory Information Management Systems (LIMS) are tools designed to manage laboratory samples, associated data, and workflows in a user-friendly interface. KTL uses the Microsoft Power Platform to build and customize LIMS to provide organization-specific functionality, including the following:
- Samples can be tracked as individual or grouped assays.
- Workflows can be established to improve processing and reduce errors.
- Data is centralized for access and quality control improvements.
Benefits: Why do you need it?
Having a reliable LIMS can help organizations to:
- Manage and control large amount of laboratory data and samples.
- Reduce time required to track samples.
- Create detailed chain of custody.
- Provide notifications and tracking of approvals.
- Attach documents and photos to samples to provide more detailed information.
- Deliver results to identified users.
Technology Used
- SharePoint or Dataverse
- Power Apps
- Power Automate

Kestrel Tellevate News / Safety
Comments: No Comments
KTL to Present on Tools to Create a Safety Culture
Look for KTL October 15-17, 2024 at UW-Madison’s 36th Annual Product Liability Conference in Madison, WI. The Conference presents current and emerging product liability prevention practices. KTL will be leading the following session as part of the Conference agenda:
Creating a Safety Culture: Tools and Strategies for Growth
Session 6 | Presenters: Will Brokaw, MS, Consultant, and April Greene, CSP, CHMM, Consultant
Safety culture is the sum total of your organization’s values, beliefs, attitudes, and actions toward safety. It is often referred to as “the way we do things around here” regarding safety. Previous research has identified key attributes or qualities that positive safety cultures share. This presentation will review these key attributes, provide real world examples, and discuss tools and strategies organizations can use to assess and improve their existing safety culture.

MECC 2024: KTL Leads Panels on Using Microsoft 365 to Manage EHS Compliance
KTL is excited to be joining the 2024 Midwest Environmental Compliance Conference (MECC) in Overland Park, Kansas, September 24-25, 2024. MECC takes a fresh, regional approach to the increasingly difficult task of environmental compliance, permitting, enforcement, and other critical environmental issues that impact Midwest facilities and institutions.
KTL will be leading the following mainstage session as part of the workshop’s technical agenda:
How to make Microsoft 365 your go-to software for managing EHS compliance – user panel
September 24, 2024 | 1:00 pm | Moderator: Joe Tell, Principal
Implementing software to manage EHS compliance sounds expensive, but it doesn’t have to be—not when most companies already have the software they need. Learn how organizations in the utility, education, and manufacturing sectors are leveraging Microsoft 365® and Power Platform® to effectively manage compliance documentation and data.
Panelists:
Tina Baker, City of Lincoln, NE Solid Waste Management Division
Autumn Gentry, Southeast Missouri State University
Emily Muth, Conagra
Brian Wanzenried, P.E., Viterra
And be sure to stop by and visit us at Booth #13. We look forward to seeing you at MECC!

Tech Corner: Reminders and Notifications Tool
Functionality: What does it do?
Virtually every regulatory agency (e.g., EPA, OSHA, FDA, USDA) and voluntary certification standard (e.g., ISO, GFSI, organic) has compliance requirements that call for companies to fulfill compliance activities by established deadlines. KTL’s Reminders and Notifications Tool is designed to help organizations make sure these deadlines are not missed. The tool allows users to receive notifications and reminders via email, Microsoft Teams, or other messaging app that action is needed, including the following triggers:
- Document submissions
- Survey/list submissions
- Permit renewals
- Assignment due date is approaching/past due
- Item or document is awaiting approval
- Many, many others
Benefits: Why do you need it?
The Reminders and Notifications Tool helps organizations to:
- Keep multi-step processes moving forward quickly and efficiently.
- More efficiently manage compliance deadlines.
- Remind assigned staff of easy-to-forget due dates.
- Share information and reports with notifications to disseminate key information regularly.
Technology Used
- SharePoint
- Power Automate
- Email, MS Teams, or other communication platform
Comments: No Comments
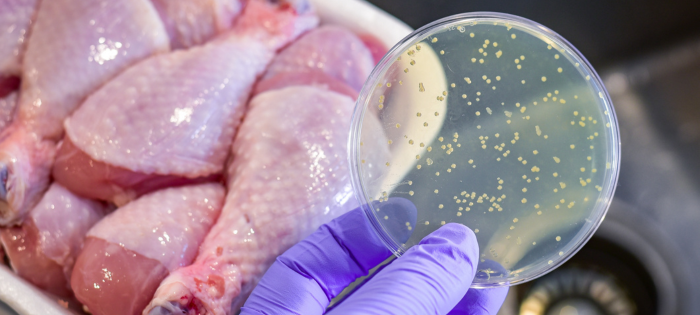
Stopping Salmonella: Proposed USDA Framework
Salmonellosis (i.e., an infection with the bacteria Salmonella) is the second leading cause of foodborne illness in the U.S. The (CDC) Center for Disease Control estimates over 1.35 million illnesses, 26,500 hospitalizations, and nearly 420 deaths annually are attributed to Salmonella. It is estimated that over 23% of those illnesses are from eating chicken or turkey.
On October 19, 2021, the U.S. Department of Agriculture (USDA) launched a comprehensive effort to reduce Salmonella illnesses associated with poultry products. Since then, the Department has completed several activities to move closer to the national target of a 25% reduction in Salmonella illnesses (see below). One of the most significant actions came on July 29, 2024, when the USDA issued its proposed rule and determination to more effectively reduce Salmonella contamination and illnesses associated with raw poultry products.
Salmonella 101
Salmonella are bacteria that live in the intestinal tract of humans and animals and can contaminate some types of food, including raw fruits and vegetables and raw or undercooked chicken, turkey, beef, and eggs. Salmonella is transmitted by consuming contaminated food products. It can also be transferred when handling pets, particularly birds and reptiles.
Controlling Salmonella contamination is tricky for several reasons. There are approximately 2,500 serotypes (i.e., distinct strains) of Salmonella bacteria, and their risks differ across all production systems. Salmonella can survive under extreme conditions and adapt to its environment. It can also survive in most animals without causing disease in the animal. In fact, Salmonella can be found just about anywhere, including in food, water, soil, and the air we breathe.
Regulatory History
USDA’s most recent proposed rule and determination represents the culmination of the Food Safety and Inspection Service’s (FSIS) three-year effort to better control Salmonella rates in poultry and protect American consumers. The following significant events have led to this latest action:
- October 2022: FSIS announces a draft regulatory framework for a new strategy to control Salmonella in poultry products.
- June 2023: The USDA Agricultural Research Service (ARS) launches the Salmonella Grand Challenge, bringing together a group of scientists from different specialties to learn more about how and where Salmonella causes the highest risk to meat and poultry products.
- April 2024: FSIS publishes a final policy to declare Salmonella an adulterant in raw breaded chicken products when they exceed a threshold of 1 colony forming unit (CFU) per gram of Salmonella contamination. This represents the first time the USDA has labeled Salmonella as a contaminating adulterant in food, alongside certain types of E.coli.
Requirements of the Proposal Rule
The July 2024 proposed framework presents a systematic approach to addressing Salmonella contamination at the poultry slaughter and processing stages. It includes the first enforceable standards for Salmonella, something that has not been established until now because previously available tools and technology were not sufficient to track the bacteria.
The rule can be broken into the following two parts:
Enforceable Limits
The proposed rule establishes final standards to prevent raw chicken carcasses, chicken parts, ground chicken, and ground turkey products that contain any type of Salmonella at or above 10 CFU per gram/mL from entering the market. It also requires facilities to test for the presence of the following, which have been deemed Salmonella serotypes of public health significance:
| Product | Serotypes* |
| Chicken carcasses, chicken parts, comminuted (ground) chicken | – Enteritidis – Typhimurium – I4,[5],12:i |
| Comminuted (ground) turkey | – Hadar – Typhimurium – Muenchen |
If the bacteria exceed the established threshold of 10 CFU per gram/mL and contain any detectable level of at least one of the identified serotypes, the poultry cannot be sold, and the product lot would be subject to recall.
Monitoring, Sampling, and Testing
The final rule also requires poultry companies to establish microbial monitoring programs (MMPs) to identify and prevent pathogen contamination throughout the slaughter system. All poultry slaughter establishments would be required to develop, implement, and maintain written procedures to prevent contamination and maintain records documenting those procedures. More specifically, they must incorporate statistical process control (SPC) monitoring principles into their MMPs to monitor the quality of the manufacturing process. Facilities must also implement written corrective actions, including root cause analysis, when MMP results differ from the predefined criteria.
Finally, the proposed standard provides for a routine sampling and verification testing program for Salmonella in chicken parts, comminuted chicken, and comminuted turkey. FSIS would sample raw final products and analyze them for Salmonella levels and serotypes to determine whether the final product is adulterated. If test results detect Salmonella at a level of 10 CFU/mL or higher and at least one Salmonella serotype of public health significance, FSIS would consider products to be adulterated and the lot would be prohibited from entering commerce or a recall would be initiated.
Moving Forward
Setting limits on Salmonella levels has been difficult until now, because existing tools and technology were not sophisticated enough to track the bacteria. USDA Undersecretary for Food Safety Dr. Emilio Esteban has stated of this proposed rule, “It’s time to change our approach…We have the tools. We have the technology. We have the knowledge.”
The USDA also has the track record. The Department took similar action with E.coli in 1994 after deadly outbreaks tied to ground beef. As a result, the number of foodborne illnesses related to E.coli has fallen by more than 50%. The ultimate goal of the framework for Salmonella is similar—to get the U.S. closer to the national Healthy People 2030 target of a 25% reduction in salmonellosis cases.
USDA is accepting comments on the proposed final regulatory framework through September 29, 2024.

Comments: No Comments
Effective Safety Leadership
Safety Focus
A strong safety culture has several characteristics in common. KTL’s research into the topic of safety culture has identified two traits that are particularly important to an effective safety culture: leadership and employee engagement. Best-in-class safety cultures have robust systems in place to ensure that each of these traits, among others, is mature, well-functioning, and fully ingrained into the standard practices of the organization.
Best Practices: Management Leadership
In its Recommended Practices for Safety and Health Programs, the Occupational Safety and Health Administration (OSHA) outlines best practices for implementing and maintaining a safety and health program. Management leadership is a core element. According to the guide, management provides the leadership, vision, and resources needed to implement an effective safety and health program. Management can exhibit this in several ways:
- Demonstrating a commitment to continuously improving workplace safety and health, including eliminating hazards and protecting workers.
- Making safety and health a core organizational value with associated safety and health goals and objectives.
- Establishing program expectations and responsibilities that engage employees.
- Providing adequate resources and support for the program
- Setting a good example when it comes to prioritizing safety and health.
- Communicating regularly with workers about the importance of workplace safety and health.
OSHA’s guide outlines four action items for management to “walk the talk” when it comes to safety and health:
- Communicate your commitment to safety and health. Management should develop a clear, written policy that demonstrates safety and health is an organizational priority. The policy should be communicated to all workers and other relevant parties, be visible in the workplace, and be reinforced by management when operating the business.
- Define program goals. These goals should focus on specific actions to improve workplace safety and health. Goals should be accompanied by assignments and timeframes to help ensure they are achieved.
- Allocate resources. Resources may include employee time, supplies, training, tools, access to safety and health experts, funds, etc. Management should integrate safety and health into the planning and budgeting process to ensure resource needs can be met.
- Expect performance. Employees need to be engaged to perform. Establish responsibilities, provide encouragement, recognize accomplishments, and set up channels for employees to speak freely about safety and health issues.
Characteristics of Strong Safety Leaders
To be a great safety leader, management must regularly demonstrate the value of safety. Strong safety leaders demonstrate the following characteristics:
- Caring. First and foremost, safety leaders care deeply about their people. Caring is about doing whatever is necessary to ensure employees return home safely every night. It involves showing concern for the personal safety of individuals, not just making a commitment to the overall idea of safety.
- Vision. Senior management sets the strategic goals and vision for the company’s safety program. Strong leaders can visualize what safety excellence looks like, how to achieve it, and how to communicate about it.
- Commitment and Action. When it comes to safety, actions truly speak louder than words. A lack of commitment, as demonstrated by action (or lack thereof), comes across loud and clear to staff. For example, requiring staff to work excessive hours or use defective parts to meet productivity goals sends a clear message that productivity is more important than safety.
- Communication. Safety leaders not only communicate the strategy clearly to the workers who carry out the company’s mission, they also incorporate safety into regular communications and engage workers in discussions about safety and health.
- Collaboration and Cooperation. Safety works best if management and workers are on the same team. Cooperation means working together to develop a strong safety program (e.g., management involving line workers in creating safety policies and procedures). It means management seeks feedback from workers about safety issues—and uses that feedback to make improvements.
- Recognition and Accountability. Effective leaders will foster the sense that every person is responsible for safety throughout the organization. They use recognition to positively reinforce safe behaviors vs. blame when incidents occur.
- Credibility and Trust. Trust in the safety program and in each other is built when leaders demonstrate these characteristics and safety is treated as a company-wide priority.
Strong safety performance is a cornerstone of any business. Management plays a key role in establishing a modeling a best-in-class safety culture where people want to work safely. These cultures can lead to:
- Fewer accidents, losses, and disruptions.
- Improved employee morale.
- Increased productivity.
- Lower workers compensation and insurance claims.
- Improved compliance with OSHA regulations.
- Improved reputation to attract new customers and employees and retain existing ones.
- Better brand and shareholder value.

Comments: No Comments
Food Chemical Safety
Safety Focus
Food chemical safety is an area that is becoming a growing public concern, especially with states including California, Illinois, and New York challenging the safety of certain food additives and other chemicals used in food. Is food safe to eat if it has chemicals?
Chemical Presence in Food
The truth is…all our food is made up of chemicals. Some naturally exist in whole foods and provide nutrition. For example, the potassium in bananas is a chemical. Some chemicals, like environmental contaminants, get into food when crops absorb them from soil, water, or air. Process contaminants (e.g., undesired chemical byproducts) can also form during food processing, particularly when heating, drying, or fermenting foods.
Chemicals may also be added to food for a variety of reasons:
- Create additional nutritional benefits (e.g., vitamins A and D being added to milk).
- Provide protection from pathogens that could make people sick.
- Enhance food by adding flavor, improving texture, and changing appearance.
- Preserve quality by preventing spoilage or extending shelf life.
FDA Authorization
The Food and Drug Administration (FDA) must authorize any chemical added to food for use as a food or color additive before it may be used, unless the substance is generally recognized as safe (GRAS). Through its pre-market review programs, FDA reviews all relevant information about the chemical before providing its authorization, including information about:
- The identity of the chemical, including its chemical structure and data on other similar substances.
- How the substance will be used, its level of use, and the amount people may be exposed to in food.
- Toxicology, safety data, and other information to show the substance is safe at calculated exposure levels.
How Much Is Too Much?
The presence of a chemical does not determine whether a food is safe to eat. Rather, it is the amount that counts.
FDA scientifically assesses the safe amount of a chemical in food by comparing how much chemical is in the food and how much someone is likely to consume daily with other safety data to determine whether a food is safe to eat. Any chemical has the potential to be harmful at a certain level, which is why this multi-pronged evaluation and extensive calculations are important.
FDA determines an Acceptable Daily Intake level for the chemical. This level has a “built-in” safety margin to ensure the allowable daily amount is actually much lower than the level known to have a possible adverse health effect.
When Food Chemicals Become Unsafe
Authorized chemicals normally used in foods as additives and preservatives can become hazardous when they are unintentionally added or are present beyond the established limits. When this happens, it can cause immediate illness and/or long-term health effects on consumers. FDA monitors the food supply for chemical contaminants and takes action when the level of a contaminant causes a food to be unsafe. Situations such as this result in food safety alerts, recalls, and withdrawals from the market.
FDA helps safeguard the food supply by evaluating the use of chemicals as food ingredients and substances that come into contact with food (e.g., packaging, storing, handling). But ultimately, food manufacturers are responsible for marketing safe foods and ensuring that they meet FDA requirements. The manufacturers are required to implement preventive controls to significantly minimize or prevent exposure to chemicals in foods that may be hazardous to human health.
More information can be found on food chemical safety at the following websites:

World Brewing Congress 2024: Don’t Miss KTL on the Food Safety Panel
KTL is excited to be participating in the 2024 World Brewing Congress (WBC) in Minneapolis, MN, August 17-20, 2024. WBC 2024 aims to provide a compass for the brewing community to chart its course through the challenges that brewers and brewing professionals encounter on a global scale.
KTL will be a panelist for the following session:
Food Safety: Requirements, Programs, Experiences & Culture in Brewing
August 20, 2024 | 10:00-11:15 am | KTL Panelist: Estefania Lopez
Beer is food. This session will introduce you to basic food safety programs and regulatory requirements needed in order to produce a safe and quality product that your customers will keep coming back to and enjoy time after time.
